Junk_Enginerd
Hazard to Others
  
Posts: 250
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
What mechanism typically stops a gas evolving reaction reaching "infinite" pressure?
Let's use aluminium in sodium hydroxide for example. If you were to mix a solution of sodium hydroxide and water, add aluminium, and then enclose it
with very little air included, the pressure would rapidly rise. I cannot imagine this pressure would be unlimited. What mechanism would stop the
pressure increase?
|
|
|
Belowzero
Hazard to Others
  
Posts: 173
Registered: 6-5-2020
Location: Member Is Offline
Member Is Offline
|
|
Just to clarify; are you saying you expect the reaction to find an equilibrium at a certain pressure ?
Also , obviously the container would give way and you are also assuming this is a perfect container being able to hold any pressure.
Correct?
|
|
|
Eddie Current
Hazard to Self
 
Posts: 78
Registered: 25-7-2018
Member Is Offline
|
|
The reactants becoming exhausted as generated products.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Assuming the reactants are not limiting I would say the pressure keeps building until the gas becomes a liquid, I don't think the reaction would stop
for some reason.
|
|
|
B(a)P
International Hazard
    
Posts: 1112
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Changing the pressure on a reaction involving liquids and solids has no impact on reaction rate so it goes until one of the reactants are totally
consumed. For the example you give, if the vessel could contain such pressure, the sodium hydroxide solution would presumably form a solid at some
pressure and the reaction would cease I imagine?
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I don't think salts will easily fall out of solution because of pressure.
Sci-hub.tw/https://doi.org/10.1021/ja01319a030
|
|
|
Junk_Enginerd
Hazard to Others
  
Posts: 250
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
Quote: Originally posted by Belowzero  | Just to clarify; are you saying you expect the reaction to find an equilibrium at a certain pressure ?
Also , obviously the container would give way and you are also assuming this is a perfect container being able to hold any pressure.
Correct? |
Yes, that was my assumption. Like the expansion pressure of freezing water being limited by the fact that once a certain pressure is reached, the
water will remain liquid because of the pressure.
Wow. So based on these replies, enclosing a NaOH+aluminum reaction with almost no air in something ridiculously strong like a 20 mm thick walled steel
cylinder of dia. 80 mm, would likely rupture or deform said container? That's fascinating.
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
There was this test at the beginning of 20th century where a very thick walled reactor was used to contain an explosion. I can't find the source but I
remember it stating that setting it off caused only loud "BLING" sound, and that's it. All the gases remained in, and could be analyzed. 20mm isn't a
thick wall at all. Let's speak of 500mm or 1000mm of high tensile strength material, like Chrome-Molybdenium, maraging steel, tungsten alloys, etc.
They are used in diamond anvils to generate megabars of pressure.
Pressures in millions of bars are routinely faced in planetary structures and material properties are somewhat well studied in ultra high pressures.
The ultimate pressure would eventually form solids from hydroxides, water will freeze, hydrogen will form a metallic lattice, etc.
I suppose this could be used for diamond anvils and other ultra high pressure reactions to generate high pressures if it would work that way. Probably
it'll stall for physical reasons far before that.
Chemical reactions are far more stronger than physics, but nuclear and quantum physics will take it to completely another level. The pressures,
whenever measurable, are in giga- or terabars, for example the core peak pressure of thermonuclear weapon. Even this won't turn matter into an
otherworldly form, and we're still far, far away from neutron stars and black holes. 
[Edited on 20-7-2020 by Refinery]
|
|
|
wg48temp9
National Hazard
   
Posts: 761
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by Junk_Enginerd  | Quote: Originally posted by Belowzero  | Just to clarify; are you saying you expect the reaction to find an equilibrium at a certain pressure ?
Also , obviously the container would give way and you are also assuming this is a perfect container being able to hold any pressure.
Correct? |
Yes, that was my assumption. Like the expansion pressure of freezing water being limited by the fact that once a certain pressure is reached, the
water will remain liquid because of the pressure.
Wow. So based on these replies, enclosing a NaOH+aluminum reaction with almost no air in something ridiculously strong like a 20 mm thick walled steel
cylinder of dia. 80 mm, would likely rupture or deform said container? That's fascinating. |
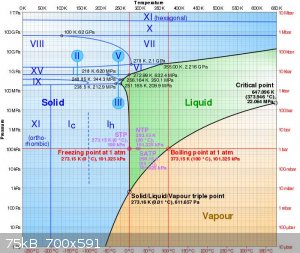
or fill the steel cylinder with water and freeze it. If your curious about what pressure ice can produce before turning back into water its about
2,000bar at -20C. See the phase diagram for water:
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 473
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
I would say the pressure builds up to the point where either the gas starts dissolving in solution and thereafter exists as a liquid assuming the
container can widthstand the pressure and some reactions even completely stop or halt at increased pressures it all really depends.
it still wouldnt build to infinity since you are putting finite reactants which can only produce a finite ammount of gas until the reaction is over.
and depending on the type of reaction it will do any of the above.
for your example of NaOH + Al the limiting action preventing pressure from reaching infinite is the finite reactants reaching a final finite pressure
once the reaction is over and hence you could say the extent of reaction and rate of reaction limit the climbing of the pressure.
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Even the reaction of NaOH with aluminum is reversible:
2Al + 2NaOH + 2H2O <--> 2NaAlO2 + 3H2
With sufficient hydrogen pressure, the reverse reaction should occur but I would assume thousands of bars are required.
A simple test chamber could be assembled from high pressure hydraulic fittings and a pressure gauge. The chamber will be an armed pipe bomb once the
reactants are exhausted, so I would also include a burst disc or a release valve that can be operated remotely to vent the system afterwards.
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
The most high pressure cartridges produce over 6000 bars of peak and the wall thickness is way less than 20mm and most of that is for kinetic reasons
and rigidity, not for durability. I don't know what would be the burst pressure of such vessel, but it could easily be in order of 20 000 bars +.
Low end explosions produce 20kbar pressure, like ANFO, and highly brisant ones 200k+. Slowmo shows that pipe bombs charged with slow explosives like
black powder, flash powder will just rip apart and release pressure, ANFO will cause it to turn into several large chunks, but actual brisants will
turn it into fine fragments of high effectiveness. It could be hence thought that even a decent container is able to actually hold lower end
pressures, if the pressure would be applied in a controlled manner.
The more interesting aspect from materials science pov is what happens when material bulk at the inner surface of the chamber goes into plastic phase,
while the mid and outer regions remain at elastic range. The metal would actually flow and form under the pressure, until it finds an equilibrium.
|
|
|
CharlieA
National Hazard
   
Posts: 645
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
If I weren't so lazy, I would make the following calculation. I would assume reacting 1 mole of Al with excess NaOH in a closed container of a
specified volume, say 0.1 L or 1.0 L. Then calculate the moles of H2 produced, and then calculate P for the given T and V. I think that
speculating that the pressure would build up indefinitely, or a solution would turn into a liquid (in this case you said the sodium hydroxide was
already in a solution), or the solute might crystallize from the solution as have been proposed variously above, are rash? or highly speculative
assumptions.
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. Try it, and see what happens. There are lots of things to be considered.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Refinery  | The most high pressure cartridges produce over 6000 bars of peak and the wall thickness is way less than 20mm and most of that is for kinetic reasons
and rigidity, not for durability. I don't know what would be the burst pressure of such vessel, but it could easily be in order of 20 000 bars +.
Low end explosions produce 20kbar pressure, like ANFO, and highly brisant ones 200k+. Slowmo shows that pipe bombs charged with slow explosives like
black powder, flash powder will just rip apart and release pressure, ANFO will cause it to turn into several large chunks, but actual brisants will
turn it into fine fragments of high effectiveness. It could be hence thought that even a decent container is able to actually hold lower end
pressures, if the pressure would be applied in a controlled manner.
The more interesting aspect from materials science pov is what happens when material bulk at the inner surface of the chamber goes into plastic phase,
while the mid and outer regions remain at elastic range. The metal would actually flow and form under the pressure, until it finds an equilibrium.
|
One of the interesting aspects of nuclear bomb design is the requirement to hold the reaction together for as long as possible.
Obviously, you can't rely on the physical strength of any material in those circumstances.
So they just use a heavy case.
The raws of physics mean that even the pressure of an atom bomb take time to accelerate a mass and move it out of the way.
For conventional explosives, I suspect the same often applies. The pressure build up is due to the time it takes to burst the container, more than its
strength.
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
The first rule of engineering is everything will break if you apply enough force.
The laws of chemistry basically state a reaction is dependent on concentration, pressure and temperature.
At some pressure the generated hydrogen becomes a solid metal, currently predicted to be above 400GPa.
Claims of solid hydrogen are currently at 425GPa using a diamond anvil.
So there is your limit.
|
|
|