Eddie Current
Hazard to Self
 
Posts: 78
Registered: 25-7-2018
Member Is Offline
|
|
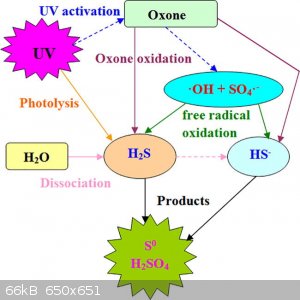
H2S + Oxone + UV --> H2SO4
Here's a novel gas scrubbing system utilising UV & Oxone which might be of interest to the Euro folks..
https://www.sciencedirect.com/science/article/pii/S138589472...
[Edited on 23-7-2020 by Eddie Current]
|
|
|
Draeger
Hazard to Others
  
Posts: 185
Registered: 31-1-2020
Location: North-Rhine Westfalia, Germany
Member Is Offline
Mood: Slowly getting ready for new projects
|
|
That sounds very useful. I'll be sure to try it out once I have a fume hood, in case anything leaks.
Maybe it'd also be a useful route to sulfuric acid?
S + [Metal] = MS
MS + [Acid] = H2S
H2S + KHSO5 + UV = H2SO4
Collected elements:
Al, Cu, Ga, C (coal), S, Zn, Na
Collected compounds:
Inorganic:
NaOH; NaHCO3; MnCl2; MnCO3; CuSO4; FeSO4; aq. 30-33% HCl; aq. NaClO; aq. 9,5% ammonia; aq. 94-96% H2SO4; aq. 3% H2O2
Organic:
citric acid, sodium acetate, sodium citrate, petroleum, mineral oil
|
|
|
Okrutnik2137
Harmless

Posts: 6
Registered: 12-9-2019
Member Is Offline
|
|
Quote: Originally posted by Draeger  | That sounds very useful. I'll be sure to try it out once I have a fume hood, in case anything leaks.
Maybe it'd also be a useful route to sulfuric acid?
S + [Metal] = MS
MS + [Acid] = H2S
H2S + KHSO5 + UV = H2SO4 |
You can make H2S directly S +Paraffin wax + heat = H2S
Regarding synthesis of the sulfuric acid even if it could be performed in an amateur lab it is much easier, cheaper and safer to just buy sulfuric
acid, if you need only small amount of it you can use an old amateur method, electrolising CuSO4 solution using lead electrodes or if you
really want to make your own acid I would rather opt for oxidazing SO2 using metal oxides, Fe2O3 works reasonably
well, hell, you can even buy V2O5 since you need only catalytic amounts.
Also, note that this paper is written by the Chinese they are known for submitting flawed or fabricated results and generally can not be trusted.
[Edited on 24-7-2020 by Okrutnik2137]
[Edited on 24-7-2020 by Okrutnik2137]
[Edited on 24-7-2020 by Okrutnik2137]
|
|
|
earpain
Hazard to Others
  
Posts: 102
Registered: 11-9-2019
Member Is Offline
|
|
Quote: Originally posted by Okrutnik2137  |
Also, note that this paper is written by the Chinese they are known for submitting flawed or fabricated results and generally can not be trusted.
[Edited on 24-7-2020 by Okrutnik2137]
[Edited on 24-7-2020 by Okrutnik2137]
[Edited on 24-7-2020 by Okrutnik2137] |
I have no qualms with observations of tendencies of cultures of nations as a whole, positive or negative. But may I ask you:
By chance do you have any references , to exemplify said flawed or fabricated results? And were the results later debunked also in scientific
publications?
Thanks
P.S. I am not Chinese.
|
|
|