Hydrazine
Harmless

Posts: 26
Registered: 21-10-2008
Member Is Offline
Mood: No Mood
|
|
68% Nitric Acid Enthalpy of Formation
Hi Guys,
I am looking for the solution enthalpy of formation on 68% nitric acid.
Any suggestions?
Thanks!
|
|
|
reactofurnace
Hazard to Self
 
Posts: 76
Registered: 17-7-2015
Member Is Offline
Mood: Volatile
|
|
You can use a table with the enthalpy change of reactions and apply hess's law.
So for nitric acid the standard enthalpy of formation would be:
1/2 N2 + 3/2 O2 + 1/2 H2 ---> HNO3
The reaction you can use is this:
NO2 + H2O ---> HNO3 + NO
:::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::
2 NO2 ---> N2 + 2 O2
2 H2O ---> 2 H2 + O2
2 NO ---> N2 + O2
:::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::
Δ(rxn)H (HNO3) = [ΔfH(NO) + 2 ΔfH(HNO3)] − [ΔfH(H2O) − ΔfH(NO2)]
Use this website to easily get Δ(rxn)H (HNO3):
http://www.shodor.org/UNChem/advanced/thermo/thermocalc.html
|
|
|
Hydrazine
Harmless

Posts: 26
Registered: 21-10-2008
Member Is Offline
Mood: No Mood
|
|
Hi Reactofurnace,
Thanks for the tips. I'll try to work it out here.
As starting points, the enthalpy of formation for water, nitric oxide, nitrogen dioxide and nitric acid are:
H2O = −286 kJ/mol
NO = +91 kJ/mol
NO2 = +33.2 kJ/mol
HNO3= −207 kJ/mol
68% w/w Nitric acid has 1.647 moles of H2O to every mole of HNO3.
This ratio suggests every molar equivalent of nitric acid is disassociated with 0.647 excess moles of water remaining unreacted.
Plugging the numbers into the equation:
Δ(rxn)H (HNO3) = [ΔfH(NO) + 2 ΔfH(HNO3)] − [ΔfH(H2O) − ΔfH(NO2)]
is
Δ(rxn)H (HNO3) = [91 + (2(-207))] − [-286 − 33.2]
Δ(rxn)H (HNO3) = [-323] − [-319]
Δ(rxn)H (HNO3) = -3.8 kJ/mol
Is this correct?
I'm not sure if I'm entering the numbers into the shodor.org link correctly.
This is the output I get for Nitric Acid and H2O:
Enthalpy Results = 90.076 kcal/mol
Gibbs Free Energy Results = 72.504 kcal/mol
The output numbers are positive but the reaction is exothermic so the enthalpy of formation should be negative. I must be using the thermocalculator
incorrectly. 
|
|
|
Maurice VD 37
Hazard to Self
 
Posts: 66
Registered: 31-12-2018
Member Is Offline
|
|
I am not sure these calculations make sense. They all suppose that pure HNO3 and pure water makes mixtures without any heat formation. And why bother
about NO and NO2 ?
|
|
|
Hydrazine
Harmless

Posts: 26
Registered: 21-10-2008
Member Is Offline
Mood: No Mood
|
|
Agreed.
I'm feeling that this more likely solved with heat of dilution.
So I've researched into heat of dilution but its still not entirely clear to me.
Most of the references discuss enthalpy to infinite dilution. Maybe this could be used to calculate dilution to 68%... but I'm not sure of how.
https://www.modeladoeningenieria.edu.ar/images/IntegracionII...
Perhaps it's the delta enthalpy between 55molar nitric acid dilution and 15molar?
-19.73-(-6.883) = -12.847 Kj/mol
[Edited on 31-8-2020 by Hydrazine]
|
|
|
Maurice VD 37
Hazard to Self
 
Posts: 66
Registered: 31-12-2018
Member Is Offline
|
|
What Hydrazine has calculated here is the heat produced when 1 mole HNO3 present in HNO3 65% mass is diluted to an infinite amount of water. It is not
the heat of formation of HNO3 65%. If the heat of dilution of pure HNO3 were known, it would be possible to calculate the heat of formation of HNO3
65%.
|
|
|
Hydrazine
Harmless

Posts: 26
Registered: 21-10-2008
Member Is Offline
Mood: No Mood
|
|
Hi Maurice,
There are many available online references to 100% Nitric Acid Enthalpy of infinite dilution.
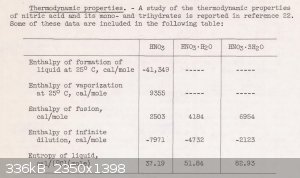
There is the link above and another reference attached. The attached table shows enthalpy of infinite dilution for 100% nitric acid. It also
includes 1 mole nitric acid with one and three moles of H2O already added in. This is very helpful to make an interpolation.
Enthalpy of Dilution
I was trying to find a direct reference or measurement of 68% azeotropic Nitric Acid heat of formation as a solution but in the absence of a reference
it can be estimated from the attached image.
The data for these three points was entered into Excel, graphed and curve fit.
The interpolated enthalpy of infinite dilution for 68% nitric acid is -4230 cal/mole.
This enthalpy loss was added to the heat of formation of pure nitric acid to get the heat of formation of nitric acid after it has been diluted to
68%.
This brings the acids ΔH to -45579 cal/mole. *This is only for the acids enthalpy, not the water.
The water ΔfH needs to be added in. After converting moles to grams and factoring in the w/w 68% Nitric to 32% H20 percentages the ΔfH can be
calculated as a whole.
68% Nitric Acid ΔfH = -1704 cal/g
Its an interpolation between points but it should be close enough for the application.
If any of you guys calculate different numbers or find a reference please let me know.
Thanks,
Tony
[Edited on 1-9-2020 by Hydrazine]
|
|
|