| Pages:
1
2 |
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
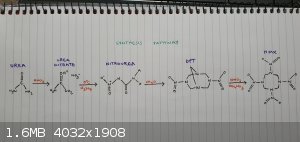
HMX via nitrolysis of DPT
1,3,5,7-tetranitro-1,3,5,7-tetrazoctane (HMX)
Formula: C4H8N8O8
Molar mass: 296.15g/mol
Density: 1.91 g/cm3
Melting point: 276-285C
VOD: 9100 m/s @ max density
OB%: -21.61
Gas volume products: 0.907 L/gram
I am attempting the synthetic pathway pictured below to synthesize HMX.
Even though some of the reagents are slightly hard to acquire (formaldehyde, nitric acid) it is more attractive as there is no need for acetic
anhydride like other methods. Being a precursor for heroin manufacture makes acetic anhydride harder to obtain than the reagents used here.
The first two steps are done and outlined in the following post.
[Edited on 4-9-2020 by greenlight]
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
So you have nitrourea? Would you mind doing a little bit of testing with it vs urea nitrate? I can't find very much info on their properties and how
they compare.
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Yes I have nitrourea but I did the steps on a small scale and only have 8 grams of it. I also suffered yield loss cause I ran out of time and
couldn't leave the reaction to stir for the required time after addition.
I do have about 500 grams of 5 year old urea nitrate though which I could turn into more nitrourea for testing. I made fresh stuff for this to
eliminate variables. In the meantime I will hunt for some properties and values for the two.
I remember testing 500g charges of urea nitrate when I was 17 using large caps and the results weren't that impressive. Similar to ANFO. Have never
used nitrourea before though.
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Urea nitrate
Not much to see here really. 22 grams of hardware store urea was dissolved in 55ml of distilled water and 30ml of 70% nitric acid added with stirring
and cooling. Filtered and dried in a dessicator. Final yield 19 grams due to not hunting for a second crop of crystals with freezer cooling.
[Edited on 4-9-2020 by greenlight]
[Edited on 4-9-2020 by greenlight]
 
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Nitrourea
reagents:
* 19g urea nitrate
* 70ml 98% sulfuric acid
method
70 ml of 98% sulfuric acid was placed into a 250ml beaker in an ice bath with stirring. When the temperature reached 0C, urea nitrate (19g) was added
in small portions while keeping the temperature low and watching for evolution of gas.
Stirring was continued for 5 minutes after last addition and then the mix poured onto ice which instantly precipitated the nitrourea as a fine white
suspension of powder.
The nitrourea was filtered, washed with ice cold water and dried in a desiccator. Yields are usually around 60-70% but my lack of continued stirring
resulted in only 8 grams which is much less at around 41%.
[Edited on 4-9-2020 by greenlight]
 
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Do you have any paper for synthesis of DPT from nitrourea ?
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Yes, I'm using this one that's actually quite old:
https://www.google.com/url?sa=t&source=web&rct=j&...
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Thanks, looking forward for your DPT synthesis. DPT itself is energetic too but never seen any test with it.
[Edited on 5-9-2020 by underground]
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Dinitro pentamethylene tetramine
Well here it is:
Materials:
* Nitrourea (7.25 grams)
* Formaldehyde solution 37% (45ml)
* Sodium hydroxide aq. (5%)
* Ammonium hydroxide (30%)
Method:
7.25 grams of nitrourea was mixed with 45ml of 37% formaldehyde solution which resulted in a white suspension. The mixture was heated to 45 C while
stirring which resulted in complete dissolution of the nitrourea.
The acidic solution was cooled to room temperature (25C) and adjusted to pH 3 via addition of 5% sodium hydroxide solution.
A small amount of carbon dioxide gas was liberated during this process.
The solution was then heated to 65C for an hour and 15 minutes. During this time, more evolution of carbon dioxide was noticed but it never got out
of control.
The solution was then cooled back to room temperature and 30% ammonia solution was added dropwise while periodically checking pH until 7 was reached.
As soon as it neared 6-7 a white precipitate started rapidly forming in the solution. At pH 7, the mixture was filtered through a Buchner funnel and
the filtrate returned to the fume hood where additional ammonia solution was added resulting in a second crop of DPT.
Filtering and further addition of ammonia was repeated but resulted in only a miniscule further amount of DPT.
Final dried weight of DPT is 3.80 grams. This corresponds with the paper used where 52% yield was noted.
Right after the nitrourea addition, my favourite (only green) spirit thermometer rolled off the bench that I have used in energetics for 10 years+
now
After all the work it has done with temp control over the years I felt like it needed a mention.
[Edited on 7-9-2020 by greenlight]

[Edited on 7-9-2020 by greenlight]
   
[Edited on 7-9-2020 by greenlight]

[Edited on 7-9-2020 by greenlight]
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Respect. Could you actually try to detonate some of this stuff? Looking forward to your HMX synthesis 
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Thanks 
Yes, I might run another batch as I was thinking it would be interesting to detonate some to see the results.
I only have 70% nitric acid at the moment so I will have to distill it atmospherically with sulphuric acid and then with vacuum to get the high
strength acid for the nitrolysis.
[Edited on 7-9-2020 by greenlight]
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Atmospheric distillation of 70% nitric acid complete resulting in yellow fuming nitric acid.
Vacuum pump is playing up at the moment resulting in yellow acid even under vacuum distillation.
Opted to try the dry air pumping method to clean it up instead.
Brought an aquarium air pump from a pond supply store and found that my (chinese made) gas washing bottle stem has broke overnight for no apparent
reason whatsoever which I have noticed is a reoccurring trend with this cheap glassware. Placed the pump in an empty desiccator and covered it with
anhydrous calcium chloride without using a drying bottle as an alternative method to pump dry air.
1 hour later and I now have 60 ml (volume has reduced) of clear high purity fuming nitric acid with no apparent nitrogen oxide(s) contamination.
Should be sufficient for the final nitrolysis of DPT.
[Edited on 23-9-2020 by greenlight]
  
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Nice job Greenlignt. I am waiting for your proress, really interesting stuff.
|
|
|
Microtek
National Hazard
   
Posts: 827
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I find that 5-10 minutes is plenty for purging the NOx from the nitric. As you have noted, you do lose some nitric doing this, so excessively long
purging should be avoided.
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Thanks underground
@Microtek, only 5 or 10 minutes? Wow, at what temperature was this done at and did you include any other factors differing from my setup?
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
Microtek
National Hazard
   
Posts: 827
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
50C, just like the urea method. I think this is a temperature sweetspot that is high enough that the NOx becomes quite labile, but low enough that not
much HNO3 is lost. In my experience, it is vey important that the air stream is as dry as possible, otherwise the NOx is not purged as efficiently (I
don't know why this should be the case).
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
I thought that 50C would start to decompose the acid as well as purge the nitrogen dioxide but 10 minutes is much more efficient considering I lost
almost 10ml of nitric acid.
I will be completing the last step today doing a test run with 1.5g of DPT using excess acid and ammonium nitrate to aid nitrolysis and hopefully
result in a good yield.
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
greenlight
National Hazard
   
Posts: 705
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
1,3,5,7-tetranitro-1,3,5,7-tetrazoctane
For the final nitrolysis of DPT, this paper was used: http://www.energetic-materials.org.cn/publish_article/2015/1...
The paper found that the NH4+ ion (not just the NO3-) has a positive effect on the nitrolysis step yield-wise when compared to other nitrate salts
such as copper and potassium nitrate. It also states a large molar excess (10:1 AN-DPT) results in high 60% yields of final product.
One thing I changed was the amount of 99% nitric acid using more than the authors of the paper did.
Materials:
* 1.5g DPT
* 20 ml 99% nitric acid
* 6.44g ammonium nitrate (10x molar amount of DPT)
Method:
6.44g of desiccator dried ammonium nitrate was mixed into 15ml of white fuming 99% nitric acid in a 100ml round bottom flask with magnetic stirring in
a salt-ice bath. The resulting pale yellow solution was cooled to about 5C which resulted inthe ammonium nitrate recrystallizing out making further
stirring impossible. Another 5 ml of 99% nitric acid was added in an effort to re-dissolve the ammonium nitrate before the DPT was added in small
portions with a spatula. White fumes appeared on each addition and the final temperature was 8.5C.
After DPT addition, the solution was left stirring in the ice bath for 30 minutes in which time the temperature dropped to 4C resulting in a semi
solid mixture of ammonium nitrate appearing again. After 30 minutes, the ice bath was replaced with a warm water bath and the mixture held at 25C for
40 minutes before being crashed into ice water to precipitate the final HMX which was filtered and washed 4 times with ice cold distilled water.
Final yield after drying is 0.9 grams from 1.5 g DPT. If working on purely weight this is a 60% yield but the molecular weight of the HMX (296.15
g/mol) is much more than the DPT (186.17 g/mol). So theoretically, the final yield should be about 2.3 grams but then you have another paper stating
1.23g from 2g of DPT is a 45% yield which would make mine a 45-50% yield but I am not sure.
I still have 2 grams of DPT left and can think of a few factors that can be played with for the last run:
1. The ammonium nitrate was ice-pack grade and probably impure which I am quite sure was responsible for the yellow discolouration. I will
synthesize my own from nitric acid and ammonia solution next time.
2. Slightly less ammonium nitrate to allow for better stirring with no solid phase at low temperature.
3. Longer mixing at room temperature before crashing into ice.
[Edited on 3-10-2020 by greenlight]
[Edited on 3-10-2020 by greenlight]
   
[Edited on 3-10-2020 by greenlight]
The only use for an atomic bomb is to keep somebody else from using one.
George Wald
|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
You have done a great job, keep up the good work man, really informative thanks for sharing
|
|
|
B(a)P
International Hazard
    
Posts: 1114
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Plus one to that. Thanks very much for such a great site up. Really interesting stuff.
|
|
|
Nitrosio
Hazard to Self
 
Posts: 57
Registered: 31-3-2018
Member Is Offline
Mood: No Mood
|
|
2C6H12N4 + CO(NH2)2 + 10HNO3 + 2NH4NO3 = 3C4H8N8O8 + CO2 + 11H2O
[Edited on 26-11-2020 by Nitrosio]
|
|
|
B(a)P
International Hazard
    
Posts: 1114
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
That would be an interesting route to HMX. Do you have any more detail or a reference where it came from?
Thanks
|
|
|
Nitrosio
Hazard to Self
 
Posts: 57
Registered: 31-3-2018
Member Is Offline
Mood: No Mood
|
|
DPT from Urea
CO(NH2)2 +2HNO3 = CO(NH-NO2)2 + 2H2O
CO(NH-NO2)2 + 3CH2O + H2O = CH2OH-NH-NO2 + (CH2OH)2-(N-NO2) + CO2
CH2OH-NH-NO2 + (CH2OH)2-(N-NO2) + 2CH2O + 2NH3 = C5H10N6O4 + 5H2O
CO(NH2)2 + 2NH4NO3 + 5CH2O = C5H10N6O4 + CO2 + 6H2O
DPT from Hexamine
(CH2)6-(N)4 + 2HNO3 = CH2(4)-(N-NO2)2-(N-CH2-N) + CH2O + H2O
HMX from DPT
CH2(4)-(N-NO2)2-(N-CH2-N) + 2HNO3 = (CH2)4-(N-NO2)4 + CH2O + H2O
|
|
|
Nitrosio
Hazard to Self
 
Posts: 57
Registered: 31-3-2018
Member Is Offline
Mood: No Mood
|
|
Attachment: DPT7.pdf (14kB)
This file has been downloaded 531 times
|
|
|
Nitrosio
Hazard to Self
 
Posts: 57
Registered: 31-3-2018
Member Is Offline
Mood: No Mood
|
|
Attachment: DPT6.pdf (14kB)
This file has been downloaded 488 times
[Edited on 5-12-2020 by Nitrosio]
|
|
|
| Pages:
1
2 |