Organicsynth
Harmless

Posts: 16
Registered: 13-10-2019
Member Is Offline
|
|
Reaction Cooling Equipment. What's out there?
I'm doing some reactions that I need the contents inside not to go over 5ºC.
I've been using a container with cold water and ice cubes but I want to do it with proper lab equipment.
The problem is that I don't know for what equipment to look.
I've tried to look for "chillers" but they don't take the temperature to 5ºC (or lower, because to keep 5ºC the water must be chilled under 5ºC).
What do you guys recommend to refrigerate the reaction flask without ice cubes?
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
The official "professional lab" way to do it is with ice.
https://www.sciquip.co.uk/products/ice-makers.html
With added salt if needed.
|
|
|
Organicsynth
Harmless

Posts: 16
Registered: 13-10-2019
Member Is Offline
|
|
Really? :O
I never thought that in professional labs chemists used ice cubes to cool their reaction flasks...
Always thought they had some kind of refrigeration machine, like a radiator that would be placed inside a water container to cool the water or
something like it...
I'm really surprised.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
I suppose it's faster to adjust and also cheaper to do it the old way.
The manufacturing guys use single or double layer mantled reactors into which a heat transfer medium that is either cooled or heated liquid is
circulated. The outer layer is vacuumed and acts as an insulator. In industry these mantles are usually not just tanks, but channels in which also
steam can be used as the medium.
As far as I know, many specialty reagent manufacturing facilities use widely these types of reactors from tens to few hundreds of liters or volume. I
wouldn't be surprised to find a number of dept's from major chemical suppliers that make batches of basically everything that can be produced upon
order with these types of setups. They are easy to dedicate into mass production, but also suitable for batch production due to their variability.
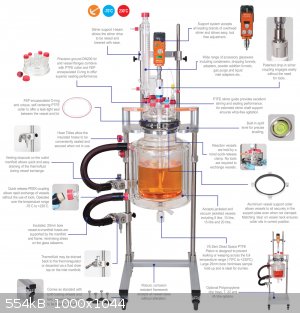
I use a styrofoam box with plastic bag lining for cool jobs and also have used it as a coolant reservoir.
I use a thermos flask for cold trap. It was still cold with solid ice floating on the water at the second day.
I was gonna consider buying a mantled reactor just for the fancy of it, but haven't yet got to do it. Pumping very hot or very cold liquids can also
require either peristaltic or other special pump.
PS. I noted that stirring the coolant bath significantly improves the cooling power of the bath. With heating baths this is not usually so big of an
issue if the thermal gradient is large, but in cooling the gradient can be very small, so little convection occurs. I used a small aquarium pump to
just move the water around, and I could run the addition phase of an exothermic reaction at least twice faster, even more.
[Edited on 20-9-2020 by Fyndium]
|
|
|
Dr.Bob
International Hazard
    
Posts: 2656
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
I work in a real lab, and we use ice all of the time. The main use for chillers is rotovap condensers. We do have one or two chillers set up for
long term cooling of reactions (overnight, or odd temps), but for many cases, ice is much easier to set up, scale up, and clean up. Other solutions
include putting reactions in the fridge or freezer overrnight (I have two there now), or other bathes like dry ice or liquid N2 in various solvents.
A real chiller will cost thousands, and you need jacketed reactors or flasks or intermediate baths to cool standard flasks. If you need a cheap
chiller with poor controls, you can take a small dorm fridge and put a bucket of water or antifreeze in it, chill it for a long time and then use a
pump to flow it to your reaction or rotovap. It may not work for hours or days, if heavily loaded, but for shorter times, it can be a poor man's
chiller. But if you get a chiller, I do have some jacketed reactors and flasks still left to sell.
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
If you can find one for cheap, get a recirculating chiller. New they cost about $5k, but you can get them on ebay for a few hundred $ sometimes.
Typical units can go between -30 °C and +100 °C and can pump a fluid through whatever container you please. Jacketed flask, beaker, reactor,
condenser, etc. Really great stuff, and the temperature stability is easily below 1°C.
At 0°C my unit can remove about 125 W of heat (See the plot below). At 15 °C, it's closer to 200 W. The temperature goes to -25 °C at which points
heat removal is 0 W.
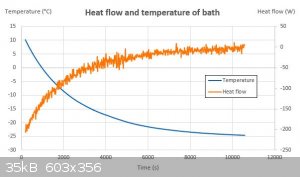
|
|
|
monolithic
Hazard to Others
  
Posts: 435
Registered: 5-3-2018
Member Is Offline
Mood: No Mood
|
|
I've run reactions inside a freezer (-20 C) and a refrigerator (5 C.) Not sure what kind of a reaction apparatus you're using but if it's just a
stirred beaker it's pretty doable. I usually submerge the beaker in a larger beaker containing pre-chilled liquid, to act as a heat buffer.
|
|
|
pneumatician
Hazard to Others
  
Posts: 409
Registered: 27-5-2013
Location: Magonia
Member Is Offline
Mood: ■■■■■■■■■■ INRI ■■■■■■■■■■ ** Igne Natura Renovatur Integra **
|
|
if you live in Alaska, Siberia... wait untill a very cold nigth in winter, -20 to -60ºC with one or two fans at full speed over the reactor 
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
This isn't even as far from a joke as one could think. I've been using snow and ice for controlling cooling bath temp many times.
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Also work in a real lab, ice water bath or dry ice / IPA bath adjusted to temp.
|
|
|
Organicsynth
Harmless

Posts: 16
Registered: 13-10-2019
Member Is Offline
|
|
That looks really badass Fyndium, it's really some next level equipment.
On most videos and talks, all we see is people filming/talking with small 500ml/1L flasks, I would really love to see big chemistry equipment being
used.
I didn't knew about those "recirculating chillers", they do look affordable and do the job exactly as it is supposed to be done, I'll take a serious
look at getting one.
It's interesting to see so many people still using ice cubes on a real lab, I suppose you're doing research type synthesis right?
I think that a real lab would not be using ice cubes to produce commercial batches of substances. I might be wrong though, with those big ass ice cube
machines... it really makes me wonder.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Commercial labs that manufacture stuff use mostly setups like I posted. They have purpose-built stations and they usually do stuff batch-wise. Every
technique that works and is economically viable is indeed used, including flaked ice, but of course the actual reactors always use pumpable mediums
and are controlled from a control room and their upper limit size can be found in places like oil refineries, etc. I have a picture that specialty
chemicals are usually produced in the two-digit liter volume range per batch, rarely in a tabletop setups in few liters or smaller, unless they are
something extremely special and expensive, and mass productions like pharmaceuticals run in the three to lower four digits. For example, sildenafil
synthesis description dealt with amounts of 60kg of this reagent and 200 liters of that solvent, etc. 50kg of finished product results in active
substance for 1 million 50mg tablets, and prior to patents expiring they were making about 20-30 dollars a piece at the counter. Similar return of
investment ratios can be achieved in the unofficial active ingredient markets, although they usually exclude the pharma grade gear, high pay for staff
and other scrutiny costs for all the legal stuff. Nowadays those blue pills are dealt though at 20-30c a piece.
But, when you think for example in 10 liter scale, you quickly do the math that the mere reagents cost over 100 euros you put in there, even when they
are bulk stuff, and you can easily top that with one extra zero if you are actually doing something more special. Make a mistake, and it can be all
down the drain. For many people, 1000€ is 2-4 weeks pay before tax. All of a sudden you also realize that you cannot simply grab the flask and swirl
it, nope, you need a siphon or a drain valve to move the reaction liquor and you most likely have to split it in multiple phases for example for
filtration, separating liquid layers, etc. When it gets big enough, you just plug in tubes, pumps and other dedicated stuff and watch as the process
liquor moves. And did I mention that normal magnetic stirring reaches its limits VERY quickly, I've done many reactions in where it was already
unsuitable and had to use overhead stirring. And then there is the heating/cooling aspect. Large volumes also pose a huge safety risks. The vapors
emitted aren't just whiffs, they are enough to incapacitate, the odors become dominating and the evaporation can also cause aerosol/gas explosion or
fire risk, and the liquor itself has a huge fire potential if something goes wrong. How about a nice runaway in a 50-liter reactor which shoots a
10-liter volcano of NFPA class 3 burning, corrosive and toxic liquid all over the place. Of course, all of this cost a LOT of money to run properly in
the first place, and most amateurs lack proper funding, hence their reactors are made from old boilers, garden hoses, buckets and bbq burners, and
these things added up with everything formerly noted are an absolute recipe for a disaster. These guys who skip the details, though, end up too often
in the news when they have burned/blown up their houses or caused large evacuations of apartment buildings. I merely have to mention what they are
usually cooking.
Just to point out a few reasons why for an amateur it is quite smart to stay in the 500mL volume, and sometimes go smaller than that.
Hence, few people actually carry out large reaction volumes unless they do it for profit. Most common amateur high volume reaction can be for example
making acetates or salt metathesis reactions, where a largish volume is initially needed, and it is then decanted or boiled off. Much, much fewer are
those that load up tens of liters of actual synthesis reagents and do the magic. Biggest reaction volume I've ever ran has been 9 liters, and it felt
a little different draining multiple cans of acetone down the stainless steel vessel, and even the minor reagents were measured in 100's of grams. 1kg
bottle of NaOH granules isn't that much after all. I used a 60-liter plastic garden bucket as a coolant bath and siphoned everything around.
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
A "regular" chiller for aquariums might do the trick if you only need 5c and it would cost you a lot less.
Let me explain:
They're rated for a certain volume of water and need a pump to circulate the water at a certain speed.
What happens if your volume is much lower and / or you circulate water at a lower speed ?
I've used one for totally different purposes so many years ago that I cant remember what was the lowest temperature it could achieve. One thing I do
remember is that it was doing it's job wonderfully.
But being compressor based it was also noisy !
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
Dr.Bob
International Hazard
    
Posts: 2656
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
But most research labs stay at 5L or below, and for that, trying to set up a jacketed reactor is a lot of pain and money. So we just use ice as
stated unless we need something unusual. It's easy to add more ice or warm the reaction by removing the bath. So it is quick to regluate it. A
chiller takes minutes to hours to change the temp, on a large scale, so it is best for processes that are well defined and previously examined on
smaller scale. More than once I have had to change the temp of a reaction quickly, either to cool it to clow it down, or to warm it to avoid it
from freezing solid, in the case of some very cold baths. More than once I have found that some solvents don't stay liquid at -78C.
|
|
|