Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Report on making bismuth oxyiodide
A synthesis of bismuth oxyiodide appears in Brauer’s handbook as well on various other places online, for example on prepchem:
https://prepchem.com/synthesis-of-bismuth-oxyiodide/
I followed the same method but with different amounts:
- 28g Bi(NO3)3.5H20 in a small beaker and add 48g glacial acetic acid.
- Stir vigorously with slight heat until all dissolved in a clear solution.
- 10.3g KI and 14.5g CH3COONa added to a beaker with 570g water and stirred until all dissolved in a clear solution.
- The bismuth nitrate solution was dripped into the bigger beaker with the potassium iodide and sodium acetate solution, while stirring.
- Each drop formed a black blotch as it entered the solution, this turned yellow within a second or 2.
- The whole solution quickly turned yellow-orange after a few ml of bismuth nitrate was dropped in, see photo below.

- The color darkened as more was added, with the final color, after all the bismuth nitrate was added, being brown-red, or as it says online “brick
red”. See photo.

- Stirring was continued for another 20 minutes and then stopped. The brick red ppt settled quickly, and the supernatant liquid was decanted. The
product was washed by 2 x 500ml portions of water by stirring and decantation.
- The solution was then vacuum filtered and the remainder washed with water in the funnel.
- The wet bismuth oxyiodide was dried on a steam bath. After some 3 hours it appeared dry, it was then powdered and dried for another 2 hours. At this
time the weight loss reduced to near zero.
- Final recovery was 19.6g. See photo of final product in bottle below.

The weight of 19.6g surprised me; I was expecting much less based on the equations on Prepchem:
1) Bi(NO3)3 + 3KI = BiI3 + 3KNO3
2) BiI3 + H2O = BiOI + 2HI
According to the above my recovery of BiOI should have been only around 7g.
I realised that if all the iodine present in the initial 10.3g of KI formed part of the BiOI (and did not proceed to also make HI) the BiOI yield
would be around 20g which made much more sense. But how to write the equation for what happened? Eventually I settled on
Bi(NO3)3.5H2O + KI = BiOI + KNO3 + 2HNO3 +4H2O
The stoichiometry of this equation agrees to the weight ratios of Bi(NO3)3 and KI stated by Brauer (and prepchem) and also the weight of the recovered
BiOI but whether it is the correct equation I do not know.
Another question I have is what is the purpose of the sodium acetate in the reaction?
|
|
|
MidLifeChemist
Hazard to Others
  
Posts: 192
Registered: 4-7-2019
Location: West Coast USA
Member Is Offline
Mood: precipitatory
|
|
Thanks for the report. It's a nice colored compound. Sorry I can't help with your questions.
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
If you have 100% yield, you obtain 20,31g. It is also possible, that your BiOI is hydrate.
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Nice job on this replication.
Bedlasky is right though. You should double check your yield calculations, because the equations that prep chem gives are more reasonable than your
proposal, and result in a theoretical yield of 20.31 grams.
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Thanks for the replies. Assuming that all the iodide from the KI ended up in the BiOI, then the 28g of Bi(NO3)3.5H2O became the limiting reagent and
the 19.6g I got represents a yield of 96%. A good yield should be possible because this product is easier to recover from the solution than many other
salts - it is heavy and the ppt settles quickly leaving a clear supernatant solution, and also when filtered the filtrate is clear. Nevertheless a
yield of 96% seems too good to be true so maybe it it a hydrate or still retains a bit of moisture.
I do think though that my results shows that all the iodine ends up in the BiOI and that no HI remains as a product.
|
|
|
MidLifeChemist
Hazard to Others
  
Posts: 192
Registered: 4-7-2019
Location: West Coast USA
Member Is Offline
Mood: precipitatory
|
|
>> or still retains a bit of moisture.
yeah, unless I bake something at 450F for a few hours, I always assume that is the case 
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by Lion850  | Thanks for the replies. Assuming that all the iodide from the KI ended up in the BiOI, then the 28g of Bi(NO3)3.5H2O became the limiting reagent and
the 19.6g I got represents a yield of 96%. A good yield should be possible because this product is easier to recover from the solution than many other
salts - it is heavy and the ppt settles quickly leaving a clear supernatant solution, and also when filtered the filtrate is clear. Nevertheless a
yield of 96% seems too good to be true so maybe it it a hydrate or still retains a bit of moisture.
I do think though that my results shows that all the iodine ends up in the BiOI and that no HI remains as a product. |
I don’t think you understand my point. When you’re using 3 equivalents of KI you can’t have all of the I end up in the BiOI,
because the ratio of Bi:I in your products is 1:3 while in your products it’s 1:1. The equations that Prep Chem gives are correct. You can write
balanced reactions til the cows come home but it doesn’t mean they reflect reality. Please try calculating the yield again, you’ll see.
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Please see attached report on making BiOI from bismuth nitrate and potassium iodide. A slightly different method using only a small amount of water
but they report the same overall equation as what I wildly guessed above.
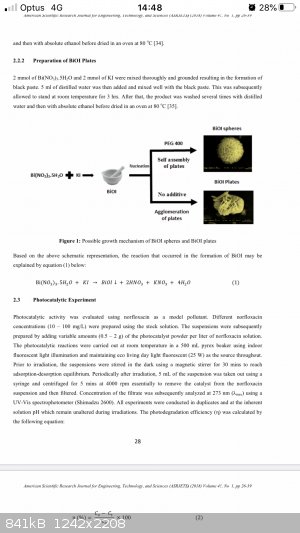
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
That’s all well and good but it’s not what you did. You used 3 equivalents of KI, and you definitely would have ended up with some HI in
the end. Your conditions were totally different. My point is that just because this equation is correct for the experiment described in this report
doesn’t mean that the equation for the report you initially followed isn’t valid. Please, calculate the yield from the equations on Prep Chem
again. Post your calculation. I did it and it came out fine. I want you to see that it works.
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Texium: 20,31 g is calculated from this equation:
Bi(NO3)3.5H2O + KI = BiOI + KNO3 + 2HNO3 +4H2O
Why HI shouldn't react with another bismuth nitrate? HI certainly reacts with bismuth nitrate. HI is also source off iodide anions. If HI doesn't
react with bismuth nitrate, you need three times more KI (so Lion should have worse yield). Equation above is overall. Prepchem equations show
mechanism, but not completly, because there is missing reaction between HI and bismuth nitrate. So finally it doesn't matter which equation you choose
for calculations.
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Bedlasky, I made an incorrect assumption. Since the procedure that Lion followed cited an equation that shows 3 equivalents of KI, I assumed that he
used 3 equivalents of KI in his reaction, and was saying that somehow all of that iodide ended up in his product. I went back and checked the amounts
he used and I see that he used roughly equimolar amounts of bismuth nitrate and KI. Thus the equations presented by Prep Chem are indeed misleading.
Apologies for the misunderstanding.
|
|
|