| Pages:
1
2 |
FragranceLover89
Hazard to Self
 
Posts: 72
Registered: 13-8-2019
Member Is Offline
|
|
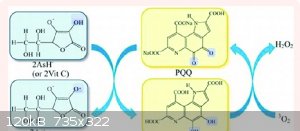
So to summarize my findings. You don't need citric acid or ammonia. All you need is L- alanine, tween 20, and zinc metal. Below I have attached two
images of the same solutions. The only difference is that the golden one (right) has zinc metal in it. The gold color is due to the reduction of PQQ
into PQQH2. Thank the chemistry gods!!! I am so fortunate to have had this beta alanine sent to me by accident after I canceled the order a few weeks
ago! What a coincidence. Ok now where is my nobel prize. Hahaha
[Edited on 30-10-2020 by FragranceLover89]

[Edited on 30-10-2020 by FragranceLover89]
[Edited on 30-10-2020 by FragranceLover89]
[Edited on 30-10-2020 by FragranceLover89]
[Edited on 30-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
|
|
|
FragranceLover89
Hazard to Self
 
Posts: 72
Registered: 13-8-2019
Member Is Offline
|
|
If any of you guys are planning on publishing this reaction I would really appreciate if you mentioned me (email me for my information). I am trying
to get into graduate school for chemistry and I really need some credit.
[Edited on 30-10-2020 by FragranceLover89]
[Edited on 30-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
|
|
|
FragranceLover89
Hazard to Self
 
Posts: 72
Registered: 13-8-2019
Member Is Offline
|
|
I have found that the concentration of tween 20 plays a huge role in the stabilization of PQQH2. Without tween 20 PQQ is reduced to PQQ semiquinone
radical, which I believe is responsible for the deep blue color the PQQ + Zinc + L-alanine solution. Adding tween 20 and a sufficient amount of water
gets rid of the blue and the pink PQQ is instead reduced to the golden color of PQQH2. Adding heat is important as well.
[Edited on 31-10-2020 by FragranceLover89]
 
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]

[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
|
|
|
FragranceLover89
Hazard to Self
 
Posts: 72
Registered: 13-8-2019
Member Is Offline
|
|
I realized that the color of my "golden solution" was a little more greenish blue than I thought suggesting the presence of semiquinone radicals. This
implied that more proton source (L-Alanine) was needed. I did not want to waste my L-alanine so I added NH4Cl and put it back on the hot plate. In a
few seconds this brought the color to the true golden color of PQQH2. Perhaps what is needed is only a catalytic amount of L-alanine, and the rest can
be NH4Cl. I also found that the blue products from earlier turned into golden PQQH2 so maybe only a super small catalytic amount of tween 20 is
needed.
[Edited on 31-10-2020 by FragranceLover89]
 
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by FragranceLover89  | | If any of you guys are planning on publishing this reaction I would really appreciate if you mentioned me (email me for my information). I am trying
to get into graduate school for chemistry and I really need some credit. This new reaction is called Yahya's reduction BTW. |
Your results might be interesting, but they need to be followed up on with further experiments and actual isolation and
characterization of products. You don't get to declare victory and claim a new named reaction just because you observed a color change.
|
|
|
FragranceLover89
Hazard to Self
 
Posts: 72
Registered: 13-8-2019
Member Is Offline
|
|
If this was 1920 I could name it. Seems like I did most of the preliminary work, which no labs would care to do without some evidence of it working. I
bet you money if you asked any chemist at MIT if they thought it was possible to replace sodium borohydride with zinc in 90% water they would laugh at
you and tell you to spend your time on research that will reliably give you results. Everyone knows the hard part is convincing your PI for funding.
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
You're missing the point: you didn't prove anything
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
/CJ
Attachment: Reduction of C=X to CHXH by Dissolving Metals and Related Methods - J.W. Huffman.pdf (2.9MB)
This file has been downloaded 386 times
Being well adjusted to a sick society is no measure of one's mental health
|
|
|
FragranceLover89
Hazard to Self
 
Posts: 72
Registered: 13-8-2019
Member Is Offline
|
|
When Bouveault–Blanc reduction was discovered in 1903 they just demonstrated it with reduction of ethyl oleate to it's corresponding alcohol. I have
not heard of direct reduction of ketones using amino acids in water. In order to get published in 2020 you need to study a couple different skeleton
structures and then diversity within those groups. Lets be honest if you get published you can call it whatever you want even if you did not discover
what amino acids work or what surfactants work or how to show that it works with a redox sensitive dye. You probably did more work than me in a
professional setting and that matters more in 2020 there is no reason to get angry.
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I have not either. And you
haven't yet proven that you've accomplished it. All you have right now is a cup of soapy yellow water.
|
|
|
FragranceLover89
Hazard to Self
 
Posts: 72
Registered: 13-8-2019
Member Is Offline
|
|
The point of using PQQ was to show that you can reduce diones to quinones. Sorry if I offended you. I thought when i said "if it was 1920 i could name
the reaction" pretty much showed I am not as serious about the name of the reaction as you.
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Quote: Originally posted by FragranceLover89  | | The point of using PQQ was to show that you can reduce diones to quinones. Sorry if I offended you. I thought when i said "if it was 1920 i could name
the reaction" pretty much showed I am not as serious about the name of the reaction as you. |
You got a colour change, you need to isolate and characterise your product before you claim you did what you said.
EDIT: I skimmed through your thread and it was very difficult to follow what you were doing. You added some turmeric? this isn't pure curcumin...
Also, the ketones you are trying are often very different, i.e. alpha beta unsaturated, conjugated systems etc.
Here is what I would do:
draw a scheme of what you are trying to achieve (by hand or with chemdraw etc)- you can also give a proposed mechanism. write down all conditions,
i.e. the number of moles, the equivalents of each reactants, the mole % of your Tween 20, reaction temperature, reaction concentration and the
reaction time.
Start with pure chemicals, weigh them accurately, do your procedure at conditions that can be easily reproduced, isolate your product and give some
sort of characterisation data. These are the three typical steps of any reaction- synthesis, isolation and characterisation. Without them, the
knowledge you are trying to produce is not any help to us.
I hope this helps...
[Edited on 31-10-2020 by HeYBrO]
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by FragranceLover89  | | The point of using PQQ was to show that you can reduce diones to quinones. Sorry if I offended you. I thought when i said "if it was 1920 i could name
the reaction" pretty much showed I am not as serious about the name of the reaction as you. |
Everything that
HeYBrO just said perfectly illustrates what I’m trying to tell you. I don’t care about the name of this reaction that may or may not even happen.
I’m not offended. I’m just trying to explain that you’ll need to research this more if you want to find out whether this can accomplish what you
think it does. You haven’t discovered a new reaction either by 2020 or 1920 standards of evidence.
|
|
|
FragranceLover89
Hazard to Self
 
Posts: 72
Registered: 13-8-2019
Member Is Offline
|
|
As a rough estimate I would say 1 ml tween 20, 8 ml H2O, 0.7 g L-alanine, 0.7 g NH4Cl, 0.07 g Zinc, 5 mg PQQ. Reaction took 15-30 minutes with
stirring. The solution got warm on the hot plate but could be held in the hand so 40-60 C? To be honest the concentration of tween in my opinion has
not mattered significantly since even a little (6 drops in 10 ml) allows the reaction to occur. It has a relatively low CMC compared to other common
surfactants like SDS. The concentration of the ammonium salt does not matter either. Just add it until no more goes into solution. Adding excess zinc
cannot hurt either since in the paper by Dash et al. they added 5 molar equivalent and they reused it between separate runs. The limiting reagent is
the ketone and or dione.
The solution goes through a yellow orange phase (in the pictures I showed) but after letting it stir longer turned a deeper orange more similar to the
vitamin C PQQ solution color.
Altogether it was very simple. Add 10/90 L-alanine NH4Cl until no more goes into solution. Add 5% by volume tween. Add some zinc and your ketone/dione
of choice. Sorry if this reaction does something random and the PQQ decomposes or something. I am sure the tween 20 can come in handy and you can just
buy some THF and follow the correct protocol in the paper by Dash et Al. Hopefully the authors are being truthful.
In the image below I compare old PQQ+vitamin c solution (sep funnel) and the zinc redox solution (on hot plate). The opaque precipitate that is a red
color is from some thing in the vitamin C tablets I am using precipitating PQQ. The clear aqueous layer has a similar orange color as the zinc redox
solution.
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]

[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
|
|
|
FragranceLover89
Hazard to Self
 
Posts: 72
Registered: 13-8-2019
Member Is Offline
|
|
This describes PQQH2 as yellowish brown, similar to the product I produced especially when you compare it to the PQQ solution I showed earlier.
http://scripts.iucr.org/cgi-bin/paper?S2052520617002281
|
|
|
FragranceLover89
Hazard to Self
 
Posts: 72
Registered: 13-8-2019
Member Is Offline
|
|
Thank you for your concerns. I hope I am not endangering my health from these experiments.
When I mixed PQQ + Zinc + NH4Cl + Tween 20 nothing happened after continuous high heating for 1-2 hrs.
When I added some L-alanine the color very quickly changed to yellow with mild heating or at room temperature. I know this is not a direct reaction
between L-alanine and PQQ, Tween, or NH4Cl because that solution is pink and turns yellow upon addition with Zn. The next possibility is that there is
an oxidative deamination requiring the presence of PQQ, Zn, and L-alanine simultaneously. This solution is a deep blue rather than the yellow seen
previously suggesting that deamination is not the common cause of color change seen in L-ala solutions with or without tween.
Research by Senda et al. 1990 show that the PQQ semiquinone radical is stabilized in aqueous solutions with 2+ metal cations. Perhaps this explains
why the variation in tween concentration caused this color difference from deep blue to yellow. My hypothesis is that Tween can complex Zn2+ or form
micelles to capture the quinol form of PQQ.
This only leaves a few more possibilities on the table. I know PQQ is not directly reacting with Zn because that solution remained pink.
Worst case scenario: L-alanine radicals are forming and reacting with PQQ to form some weird compound?
Things I have tried
PQQ + Zinc + NH4Cl (saturated) + Tween 20 Pink (Baseline) stays even with heating
PQQ + ammonia Golden color
PQQ + citric acid Golden color
PQQ + Zinc + NH4Cl (saturated) + L-alanine Deep blue color
at room temperature
PQQ + Zinc + NH4Cl (saturated) + L-alanine + Tween Bright Yellow color at room temperature or with heating depending on NH4Cl
concentration
What to try next
Zn + L-alanine + Tween 20
Zn + NH4Cl + L-alanine + Tween 20
Catalytic amount of L-alanine
Addendum
I have read that [Zn(ala)2] is colorless.
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
[Edited on 31-10-2020 by FragranceLover89]
|
|
|
FragranceLover89
Hazard to Self
 
Posts: 72
Registered: 13-8-2019
Member Is Offline
|
|
Your hunch was correct. Someone has already done this reaction and it is not producing PQQH2. The yellow color is an oxazole
[Edited on 31-10-2020 by FragranceLover89]
Attachment: j.1432-1033.1989.tb14894.x.pdf (740kB)
This file has been downloaded 226 times
|
|
|
FragranceLover89
Hazard to Self
 
Posts: 72
Registered: 13-8-2019
Member Is Offline
|
|
Ok sorry my earlier posts are kinda crappy. I was just experimenting around and thought I had the right product. I was wrong. PQQH2 should precipitate
out of solution as a brown material as described in this paper by Ikemoto et al. That is the brown material I saw when I mixed an excess of vitamin C
with PQQ earlier. In addition I was very wrong about that bright yellow product being PQQH2. It is actually an Oxazole formed by reaction between
L-alanine and PQQ in the presence of Zn2+. The paper I attached above describes what was observed pretty well. I believe there is still hope for tween
20 + Zn + NH4Cl as a reducing agent for ketones. I have attempted the reaction again with better descriptions of the reagents.
PQQH2 synthesis pt 2:
I opened a capsule of 10 mg stated PQQ and dissolved it in 33 ml of water with gentle stirring for 10 minutes to create a 0.0009177 molar solution(Pic
1). While I waited for the pill filler to settle out I added 1 drop of tween 20 to a glass container with a significant amount of zinc powder (Pic 2).
2 ml of water were left over in this container from when I washed the zinc powder with water earlier. I added NH4Cl until no more would dissolve in
the solution. To this I added 6 ml of the PQQ solution prepared earlier (5.5*10-6 moles).
The color changed instantly to a brown yellow color (pic 3).
Next I will try to determine the volume of 1 drop of tween and repeat the experiment with fresh zinc weighed out to see if this was the result of
L-alanine residue.
Attachment: ikemoto2017.pdf (882kB)
This file has been downloaded 259 times
  
|
|
|
FragranceLover89
Hazard to Self
 
Posts: 72
Registered: 13-8-2019
Member Is Offline
|
|
Using the same pipette I used add a drop of tween 20, I found there are 43 drops per 1 ml so 1 drop is .02 ml. This means that the reaction above
contained a 0.3% (solute/solution) tween 20.
|
|
|
FragranceLover89
Hazard to Self
 
Posts: 72
Registered: 13-8-2019
Member Is Offline
|
|
It is hard to see but in the 20 minutes since this reaction started. A red-brown precipitate has begun to form in the liquid.

|
|
|
FragranceLover89
Hazard to Self
 
Posts: 72
Registered: 13-8-2019
Member Is Offline
|
|
As a quick update all the PQQ has formed into an insoluble copper red precipitate with no pink left in solution. Here is an image of this precipitate
in a pipette.

|
|
|
FragranceLover89
Hazard to Self
 
Posts: 72
Registered: 13-8-2019
Member Is Offline
|
|
When the initial PQQ solution was saturated with NH4Cl the color changed from pink to peach, however, no precipitate was noted. This means the orange
precipitate is either PQQH2, the zinc complex of PQQ, or something else. Pic below is a PQQ NH4Cl solution without precipitate.

|
|
|
FragranceLover89
Hazard to Self
 
Posts: 72
Registered: 13-8-2019
Member Is Offline
|
|
The addition of a Zinc acetate solution was added to the PQQ NH4Cl solution. A white precipitate was observed.

|
|
|
| Pages:
1
2 |