Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
CuS isn't actually copper(II) sulfide
Hi.
I came across an interesting information. CuS isn't actually copper(II) sulfide. This compound contains just copper in I oxidation state. Look at
this: https://en.m.wikipedia.org/wiki/Copper_monosulfide
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Wow. Whodathunkit?
It's not going to stop me using it as an example when teaching students how to write ionic formulas. But I probably use lots of compounds that don't
actually exist.
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Many compounds are written as simple ionic compounds, like for example FeO. But in real it have complex structure and even Fe:O ratio isn't 1:1,
because FeO have oxygen vacancies.
Fe(III) have similar oxidation properties as Cu(II). When you add Fe3+ in to Na2S solution, you obtain mixture of FeS, FeS2 and sulfur. So it make
sense that Cu(II) is also reduced by sulfide.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
@j_sum1: That's the difference between high school level chemistry and chemistry at a more detailed level. I have come across many examples, where
high school chemistry is a strong simplification.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Attempting to describe CuS as an ionic compound when Cu has electronegativity 1.9 and sulfur 2.6 doesn't make sense. It's less polar than CO2. What's
important is that it contains covalent sulfur-sulfur bonds.
Really there are two motifs here:
- a hexagonal plane with stoichiometry CuS where every sulfur is bonded to three coppers and every copper to three sulfurs
- "ribbons" of CuSSCu polymer forming -Cu-S-S-Cu-S-S- hexagons which are joined on the S-S edge
The ribbons stack diagonally into sheets which are interspersed with the planes. The whole structure is covalent.
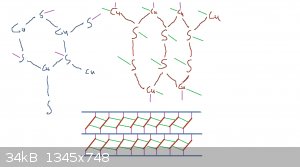
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
MidLifeChemist
Hazard to Others
  
Posts: 192
Registered: 4-7-2019
Location: West Coast USA
Member Is Offline
Mood: precipitatory
|
|
It's a similar situation with Sb2S5, the Antimony is actually in a +3 state. reaction with HCl produced SbCl3
https://sci-hub.scihubtw.tw/10.1016/0020-1650(69)80231-x
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Quote: Originally posted by woelen  | | @j_sum1: That's the difference between high school level chemistry and chemistry at a more detailed level. I have come across many examples, where
high school chemistry is a strong simplification. |
Agreed.
At the level where ions are first introduced, it makes sense to give examples involving anions and cations with different valencies. At that early
stage, polyatomic anions can add unhelpful complexity. So, having initial problems and examples using fluoride, chloride, bromide, iodide, oxide,
sulfide, nitride and phosphide really reinforces some critical patterns with respect to nomenclature and the groups on the periodic table. And this
means there is a basis to launch students into writing ionic formulae.
I am aware that many such examples don't exist or behave in real life exactly as stated. And my goal is always to move on to more realistic compounds
that students can observe and handle. It is just that MgSO4·7H2O is not a simple starting point. A slight oversimplification in theory for a class
of 13 year olds can be useful even if it means correcting the picture a little later down the track.
As a rule, I intensely dislike it when I hear myself state something that does not conform to reality. It is really easy to talk about such species
as mercury (I) fluoride to answer some textbook question but I would much prefer to use as examples things that are realistic. As times it is
expedient to make compromises and avoid divulging the whole truth.
One of the problems is that we want students to learn patterns and principles underlying chemistry. But so often in chemistry, the pattern works for
the first two or three terms and then there is an exception caused by some other more advanced principle becoming significant. (Electron
configurations for the 3d elements is a case in point.) It is not always easy to assess the level of detail that is needed to get individual students
to click without compromising their later learning.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by j_sum1  |
As a rule, I intensely dislike it when I hear myself state something that does not conform to reality. It is really easy to talk about such species
as mercury (I) fluoride to answer some textbook question but I would much prefer to use as examples things that are realistic.
|
I've learned to assuage my sense of honesty by adding, "At least as far as this course is concerned!" when I tell my students that arsenic is a
non-metal, or that silver only forms cations with a charge of +1.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Draconic acid: Most common form of arsenic is grey arsenic, which is semi-metal. Yes, it also have nonmetal form yellow arsenic As4, but it's
unstable.
But from chemical point of view you are right. Arsenic behaves in chemical reactions more like nonmetal.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
@j_sum1, DraconicAcid: I know the dilemma between educational value and realistic value. I actually have to teach things frequently in my work,
IT-related things, sometimes also some mathematical background. And with these subjects there is the same issue as with chemistry.
In my website, I also sometimes struggle with how far I should go with a page. Sometimes, if you superficially observe some phenomenon, it nicely fits
high school level chemistry, but if you go into more detail, then things become more complicated. A nice example I had in a practical situation with
explaining the reaction between I2 and SO2 in water to a young, but very eager, person, was the following:
- Prepare a solution of I2 in water. This is nearly impossible, but to help dissolve the I2 add some KI. If that is done, then the I2 easily dissolves
and you get a brown solution.
- In a separate beaker, prepare some SO2 from Na2SO3 and an excess amount of dilute H2SO4. Nice part of the demo is to let people carefully smell some
SO2.
- Mix the two solutions (assure that excess SO2 is used). You get a colorless, or very pale yellow solution. The brown color of I2 disappears.
High school explanation: I2 + SO2 + 2 H2O --> 2 HI + H2SO4 (or as ions: I2 + SO2 + 2 H2O --> 4H(+) + 2 I(-) + SO4(2-))
First more advanced concept: Why does I2 dissolve much more easily in a solution of KI? Here comes the explanation of complex ions, in this case
I3(-). At high concentration things are even more advanced, you get ions [I.nI2](-), with n ranging from 1 to 4. The color of this kind of ions is
dark brown.
A somewhat better explanation now is: I3(-) + SO2 + 2 H2O --> 4H(+) + 3 I(-) + SO4(2-)
If you really perform this experiment and you are a careful observer (this was something which for a long time puzzled me), then you see that the
resulting solution is not perfectly colorless, while all reaction products in the above equation are really colorless. The resulting solution is pale
yellow. Even when quite some excess amount of SO2 is used, you get a pale yellow solution, while you can clearly smell the SO2.
I have been looking for an explanation for this phenomenon for years, but finally, with the help of some Pakistani student who had access to an
obscure Russian journal from the 1960's he found an old paper about I(-)/SO2 adducts. A complex is formed of the form [I.nSO2](-), with n depending on
temperature and concentrations. The value of n usually is 1, but at high concentrations it can be higher than 1. This complex has a deep yellow color
and it exists in equilibrium with the free ion and free SO2. Years later, I read in an old Dutch book from Holleman, from the 1910's, that liquid SO2
is a nice solvent for several salts and that solutions of KI in this solvent are deep yellow!
So, it is a matter of depth of theory, but also a matter of how far one can go with pursuing more detailed observations.
A similar thing I found in my experiments with second row and third row transition metals. At first glance, observations seem according to theory in
textbooks, but when looking in more detail, e.g. colors are not exactly as described as in literature, or other effects are observed, such as
cloudiness of the solution.
|
|
|