Delta-R
Harmless

Posts: 16
Registered: 6-5-2018
Member Is Offline
|
|
Loudwolf “Sodium Thiosulfate, anhydrous”: WHAT IS IT?!
So I was doing a NalCO titration with KI and Sodium Thiosulfate as outlined in “Small scale synthesis of Laboratory Reagents” by Lerner and I was
getting a clear solution with barely any Thiosulfate. Ran it 3 times and was getting less than 1% NaOCl when I knew the solution was at least 8%.
So as I was looking at the label, under “sodium thiosulfate, anhydrous” was Na₂S₃O₄, instead of Na₂S₂O₃. Huh? So I popped it into
google and pretty much all the results are for various pages selling the loudwolf product, and a couple other equation balancer websites that had the
name for that formula blank.
So I decided to take a quick melting point on it. It should be 43°C according to my resources. At around 60°, there was very very slight, almost
imperceptible melting of some component, and again at 100-110, although that could’ve just been things shifting from heat or moisture. Even up to
350°, it looked unchanged. Finally at over 400°C, it started started melting into a weird green sludge (along with my melting point apparatus...).
The first picture is at around 60 degrees, the second and third are just over 400 and 430 respectively, as the green melt formed.
I decided to do a sanity check with a torch and even in direct flame, it just started to char and burn with a very sodium orange flame, and when the
torch was removed, it continues burning with an orange and purple flame. Afterwards, it leaves yellow crust so obviously it contains at least sodium
and sulfur.
The real question is just what exactly are they selling? It’s obviously a reducing agent that reacts with NaOCl. It obviously contains sodium and
sulfur, but yet it has a 400+ degree melting point.
Any guesses?
   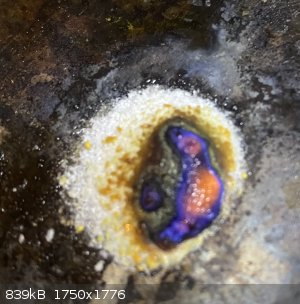 

[Edited on 12-12-2020 by Delta-R]
|
|
|
MidLifeChemist
Hazard to Others
  
Posts: 192
Registered: 4-7-2019
Location: West Coast USA
Member Is Offline
Mood: precipitatory
|
|
It looks like sodium thiosulfate decomposes at around 300 degrees celsius.
https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-thiosulfate...
|
|
|
teodor
National Hazard
   
Posts: 872
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
I won't be sure in a product of manufacturer who doesn't know the formula of the product. Sodium thiosulfate is Na2S2O3.
There are several good qualitative tests for thiosulfate ion which I think more appropriate than the melting point determination for this compound
because it decomposes on heating. They are well described in a literature, but I personally very like reaction with barium chloride, it a kind of
"delayed" reaction - see, for example in the article I attached here.
If you don't have it you can easily prepare thiosulfate by dissolving sulfur in boiling sodium sulfite solution followed by crystallisation. Some
ventilation during the process is needed.
[Edited on 12-12-2020 by teodor]
|
|
|
Deathunter88
National Hazard
   
Posts: 508
Registered: 20-2-2015
Location: Beijing, China
Member Is Offline
Mood: No Mood
|
|
Nothing wrong with it. 43C is for the pentahydrate, you have the anhydrous version which melts with decomposition at around 300C which you observed.
Occams razor says its a simple typo on the label, not some previously unheard of chemical.
|
|
|