ChemichaelRXN
Hazard to Others
  
Posts: 103
Registered: 7-10-2010
Member Is Offline
Mood: Universal Eye
|
|
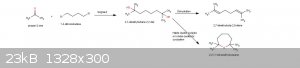
2,2,7,7-tetramethyloxepane
Hello,
Has anyone ever performed a Grignard reaction where you are reacting two halogen groups with two moles of the ketone? Using the compound
1,4-dibromobutane and acetone or even MEK.
The compound Id produce would be 2,2,7,7-tetramethyloxepane, somewhat similar to 2,2,5,5-tetramethyltetrahydrofuran (TMTHF), naturally found in the
mycelium of Tuber borchii (truffles). I’d try to use H-Beta-zeolite for the cyclization of 2,7-dimethyloctane-2,7-diol.
I think it would be a fun couple step reaction to do, but I want to know what to expect in the Grignard. Think it would spontaneously combust?
You are the same perception looking out, from the same elements around the universe.
You are everything to be anything to begin with.
Https://you-are.space
Https://syntharise.com
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Ask Henry Gilman! :-)
He studied Grignard reactions extensively and compiled a good article on them in "Organic Reactions" in the 1940-ies. He even mentioned the reactions
of alpha,omega-dihaloalkanes in that article... As far as I remember his verdict was this route was OK and went more or less according to theory.
So I encourage you to pursue this route, your target compound seems (smells?) gorgeous! :-)
|
|
|
ChemichaelRXN
Hazard to Others
  
Posts: 103
Registered: 7-10-2010
Member Is Offline
Mood: Universal Eye
|
|
Yea, it might just work. I recall hearing anything less than the 1,4-dibromobutane would just break apart. I will have to wait until the summer before
I can do this. I dont handle anything flammable with a low boiling point like THF or ether indoors in the fume hood and would do this outside. I have
the 1,4-dibromobutane and pretty much everything else luckily. Apparently that dihaloalkane is bad for the eyes, which makes me a little lazy about
handling it.
Yea, the odour must be reminiscent of the 2,2,5,5-tetramethylTHF. I would definitely document the product well if I achieve success with the final
cyclization.
You are the same perception looking out, from the same elements around the universe.
You are everything to be anything to begin with.
Https://you-are.space
Https://syntharise.com
|
|
|
|