RustyShackleford
Hazard to Others
  
Posts: 195
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Preparation of ethylene diamine from glycol and urea (via imidazolidone type polymer)
Some months ago i did some experimenting with preparing ethylene diamine from glycol and urea. I used this as a source for the preparation of the
precursor: "ETHYLENEUREA. II. Syntheses from ethylene glycol or ethanolamine and urea (or carbon dioxide and ammonia)" - Carl E. Sweitzer
I was able to obtain a 18% yield, but there are surely large improvements to be made.
Here is the way i did it:
1mole car grade glycol, 4 moles urea heated for 7 hours according to temperature chart in paper. Ammonia and CO2 escape
The hot material was poured onto a dish to cool, the glassy material is powdered after cooling by hammer and mortar. (yield ca 130g).
Material is refluxed in water (total vol 450ml) with 2 equivalents NaOH, assuming the material is on avarage imidazolidone 86g/mol (really its a
polymer) time ca 3h, you should go longer though
liquid is ideally filtered ( i didnt) and then distilled without fractination to yield crude solution of ethylene diamine, ammonia and water.
The distillate is acidified with HCl, then evaporated down untill ca 60ml from 500ml distillate
The concentrated solution is basified with NaOH, refluxed for 2-3h or untill smell of ammonia is weak or entierly covered by the amine. This is done
to remove ammonia contamination
concentrate is fractionally distilled to yield 20% (determined by titration) solution of ethylene diamine which comes over at 110-125C
If you wanted to, you could acidify this with HCl, evaporate untill dry, then distill from NaOH without solvent, this would yield much higher
concentration, likely around 80-95%, depending on water amount in the hydroxide.
Now for some notes:
Acid hydrolysis of the glassy polymer failed almost entierly (the couple drops of yield was likely produced with the basified simple distillation).
This indicated that the ethylene diamine produced comes not from imidazolidone, but from polymer, as imidazolidone is easily acid hydrolysed to yield
near quantitative Ethylene diamine.
Foaming during distillations and reflux is a problem, so dont follow my lead and use glassware filled to 80%.
Damage to glassware is possible with hot solutions of hydroxide with lots of solid, using steel apparatus would be ideal (also easier to scale up in
steel)
Im certain the product formed is specifically ethylene diamine, as i have made a half dozen complexes with it and everything has went as it should.







[Edited on 16-12-2020 by RustyShackleford]
[Edited on 16-12-2020 by RustyShackleford]
[Edited on 16-12-2020 by RustyShackleford]
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
OTC ethylene-diamine? Not bad for a first post. :-) Congratulations and welcome aboard! ;-)
What is the exact reference of the preparation you followed?
What is (are) the reaction equation(s)?
Anyway, impressive!
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 195
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Quote: Originally posted by Pumukli  | OTC ethylene-diamine? Not bad for a first post. :-) Congratulations and welcome aboard! ;-)
What is the exact reference of the preparation you followed?
What is (are) the reaction equation(s)?
Anyway, impressive! |
Thank you! i have some more exciting stuff to post too, just have to collect the images and notes to write.
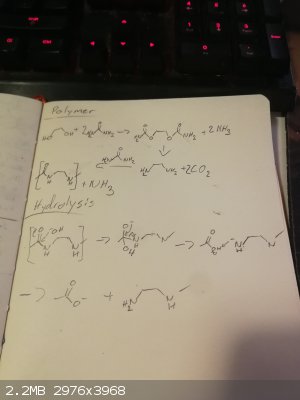
I believe the reaction proceeds by:
1: condensation of glycol with 2 urea to produce carbamate diester ,releases 2 x NH3
2: decomposition of the carbamate to ethylene diamine and 2 x CO2
3: condensation of the ethylene diamine with urea to produce stable glassy polymer
then the hydrolysis is simple amide base hydrolysis, forming free ethylene diamine and carbonate.
i attached the paper i referenced in the post in this comment
Attachment: ethylenediamine-from-ethyleneglycol-urea.pdf (440kB)
This file has been downloaded 416 times
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Nice!
extension: form mega-hexamine (1,3,6,8-tetraazatricyclo-[4.4.1.13,8]dodecane (TATD))by rxn with formaldehyde and then convert this to TMEDA
by rxn with formic acid:
https://www.mdpi.com/1420-3049/12/7/1471/htm
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Welcome to SM! Quite a first post and certainly an interesting and useful compound. It sounds like your manipulation of the intermediate stages could
do with refining as it appears that manipulation error may have caused significant losses. A nice detailed right up too so other can follow it and
undrstand why you did thing the way you did. Well done.
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 195
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Quote: Originally posted by Boffis  | | Welcome to SM! Quite a first post and certainly an interesting and useful compound. It sounds like your manipulation of the intermediate stages could
do with refining as it appears that manipulation error may have caused significant losses. A nice detailed right up too so other can follow it and
undrstand why you did thing the way you did. Well done. |
Thank you so much!
I agree that there are big improvements to be made, the reflux to digest/hydrolyse the polymer seems to be incomplete after the 3h (goopy solid that
starts to precipitate as you distill it later), so longer would certainly improve things. Also i believe that its possible a significant amount got
stuck in the thick precipiate/fluid mixture during the simple distillation, so perhaps breaking it half way, waiting for the solution to cool and
filtering it, then distilling the fluid would be better.
If you had the ability to, vacuum distilling the polymer goop, as per the paper, would allow you to work with far better quality substrate. The
biggest pain of the preparation is the distillation and hydrolysis reflux, so making those parts more efficient by using higher quality substrate
would be great.
[Edited on 16-12-2020 by RustyShackleford]
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 195
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Another way to do maybe this way better, would be to heat the glycol and urea under vacuum and capturing the gases into acid, that way it may be
possible to capture the ethylene diamine that forms and then condenses in the reaction, before it has the time to form the polymer goop.
I havent heard of anyone doing that though so cant say it works
[Edited on 16-12-2020 by RustyShackleford]
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Very intersting preparation.
It strongly reminds me of preparation of amides from acids and urea. First, N-acyl urea is produced at lower temperatures, then at higher temperatures
it is thermally converted into an amide.
However, the last step still surprises me  . .
ps. the term "imidazolidone" is rather an abuse in this case
Слава Україні !
Героям слава !
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 195
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
I agree lol, but i dont really know how polymer naming schemes work. I just put that in the title because its sort of like 2-imidazolidone except the
ethylene diamine bridges between 2 ureas instead of between the nitrogens on 1 urea. Also the stoiciometry for the hydrolysis would work out as if it
was 2 imidazolidone.
|
|
|