Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Iodide/sulphur dioxide adducts, BaI2 x 4 SO2
An interesting fact that seems to be quite poorly known is the fact that iodides of the alkali and earth alkali metals form yellow to orange-red
adducts with sulfur dioxide.
I have prepared some barium iodide by boiling a solution of barium hydroxide (made from barium chloride and NaOH, the octahydrate is poorly soluble in
the cold) with solid iodine (where the hydroxide was in excess). Barium iodate is also formed and precipitates, the solution is left to cool off, then
filtered and then evaporated to dryness. A slightly off white solid remains, BaI2.
Next, a sulfur dioxide generator is set up from dilute sulfuric acid and sodium metabisulfite. Some barium iodide is placed in an ampoule which is
placed in a salt/ice bath, and a glas pipette connected to the gas generator is placed inside. Then the acid is allowed to drip onto the bisulfite and
sulfur dioxide is liberated. First the iodide turns a lemon yellow color like that of potassium chromate, and as more sulfur dioxide is passed into
the ampoule the barium iodide turns bright orange like the color of potassium dichromate. I then flame sealed the ampoule, although over time sulfur
dioxide pressure will build up inside so maybe that wasn't such a good idea.
The yellow color is probably from the adduct BaI2 · 2 SO2 and the orange color is probably the BaI2 · 4
SO2 adduct.
It's said to also work with other iodides but barium seems to be the most stable one of them.
They sadly all decompose at fairly low temperatures and probably lose sulfur dioxide readily.
But it should be noted that even when they are stored in a way that prevents loss of sulfur dioxide they slowly decompose forming the metal sulfate,
sulfur, sulfur dioxide and iodine. Nonetheless, this is an interesting and surprising experiment, since neither of the components are known to form
colored complexes in this form, and seeing how brightly orange the compound is one has to wonder what sort of bonding makes it so vibrantly orange.
Barium iodide:

The adduct:

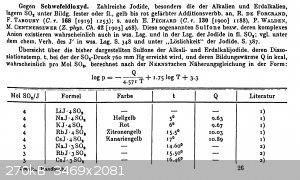
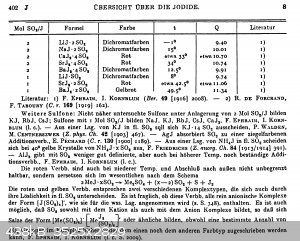
Some literature in German, it's from Gmelins handbook of inorganic chemistry, iodine part 2 ("compounds of iodine", 1933, reprint from 1955), pages
401-402
[Edited on 28-12-2020 by Diachrynic]
we apologize for the inconvenience
|
|
|
valeg96
Hazard to Others
  
Posts: 253
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
Interesting, simple and nice. I'm not a fan of making chemicals that don't last, but this is something I didn't know
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I discovered this myself, 15 years ago. I wrote a webpage about this:
https://woelen.homescience.net/science/chem/riddles/iodide+s...
PS: Does this webpage render correctly? Since a year or so, I seem to have issues with the character encoding of some webpages, but never heard
anything of this from other members. Any feedback on this would be welcome.
[Edited on 28-12-20 by woelen]
|
|
|
valeg96
Hazard to Others
  
Posts: 253
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
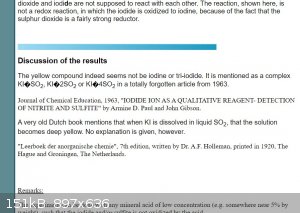
Kinda does, and changes font quite suddenly.
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
I totally forgot that you had that experiment on your website. Very nice, then it seems to work even in solution. The yellow color is somewhat
distinct from the orange color I had, but as mentioned it passed through a lemon yellow color before turning orange.
Your website looks the same to me as in the screenshots from valeg96. There are some weird unicode characters in there, and they do appear on
practically every page that has chemical equations.
we apologize for the inconvenience
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Hmm... this is not good. Nearly all pages are broken 
I fixed this single page. These pages were written years ago, and nowadays, browsers use another default character encoding. I need to change the
characters in the webpages (which must be done manually, by opening all of them in an editor) and I have to save them with UTF-8 character set
encoding. Total number of affected web pages is appr. 600 
I already made a script for setting the UTF-8 character encoding in all HTML pages, but changing the special characters probably will be a lot harder.
I use quite a few different special characters.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2694
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
time for XRD :p
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
That sucks woelen! A massive problem seems to also be that all the weird characters are the same, even tho they clearly originated from different
symbols. Sometimes what used to be a degree sign, sometimes a center dot and sometimes a minus sign, they all end up as U+FFFD. Maybe some code could
guess from context clues what the character used to be?
| Code: |
For Each i As Match In Regex.Matches(Str, "([^\s])*\uFFFD([^\s])*")
If Regex.IsMatch(i.Value, "(\uFFFD)C") Then
'It's a degree
'Regex.Replace(i.Value, "(\uFFFD)", "°")
ElseIf Regex.IsMatch(i.Value, "(\uFFFD)[A-Za-z]*?(</font>)*?</sup>") Then
'It's a minus
'Regex.Replace(i.Value, "(\uFFFD)", "-")
Else
'It's a center dot
'Regex.Replace(i.Value, "(\uFFFD)", "*")
End If
Next |
Something along those lines. So far I have only found degrees, minus and center dots to be affected, if there are more they should be added there.
Very crude method however, and some very dirty code.
we apologize for the inconvenience
|
|
|