DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
Preparation of 3,4,5 trimethoxyphenylpyruvic acid
Some time ago I reported on the preparation of an oxazolone (aka azlactone) prepared from 3,4,5 trimethoxybenzaldehyde.
https://www.sciencemadness.org/whisper/viewthread.php?tid=84...
I have been experimenting with many derivatives of this compound, including a phenylpyruvic acid (formal name:
2-oxo-3-(3,4,5-trimethoxyphenyl)propanoic acid), which in turn can lead to other interesting compounds.
Much of the procedure below has been derived from "Synthetical Experiments in the isoFlavone Group Part IV. A synthesis of 2-Methylirigenol" (Baker,
Robinson, 1929)
The reaction is summarized thusly:
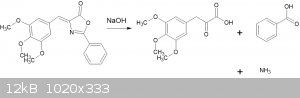
The reaction itself is straightforward, it is the separation of the phenylpyruvic acid and benzoic acid that is somewhat tedious.
__
7.78g of the azlactone (MW = 339.34 -> 22.9mmol) is added to a solution of 5g NaOH in 30ml water of water and refluxed with magnetic stirring for 1
hour. The azlactone dissolves in the first 5 - 10 minutes. After completion of the reflux, an ammonia smell is noted.
The resulting solution is a mixture of benzoic and pyruvic acids. To separate them, SO2 is passed in, forming (presumably) a soluble
sulfite adduct on the aldehyde of the pyruvic, but the benzoic acid remains insoluble in the acidic solution.
The pyruvic acid/benzoic acid solution is diluted to approx 120ml. The aim is to produce a saturated solution, so with the solubility of
SO2 being approx 94g/l, approx 0.22mol is required. To produce this, a 2-necked flask was charged with 24.5g (0.11mol) potassium
metabisulfite, and fitted with a dropping funnel with 37ml of 6M HCl (0.22mol). One neck of the flask was fitted with a tube connection, and the gas
passed into a conical flask containing the pyruvic/benzoic mix, loosely stoppered with glass wool. A magnetic stirrer was also used.
A small positive pressure of N2 was applied through the top of the dropping funnel, adjusted to result in a small amount of bubbling in the
solution prior to the addition of the HCL. With addition of the HCl dropwise into the potassium metabisulfite, and some gentle heating, plentiful gas
was generated. (This of course must be done with good ventilation.)
After several minutes, a scummy precipitate of benzoic acid begins to form. After SO2 injection has completed, the thick slurry of benzoic
acid and dissolved pyruvic acid adduct is stirred for several more hours.
The resulting mixture is filtered and the solids rinsed with water.
The filtrate is then heated and treated with HCl. The original paper calls for treating with "excess HCL" - it is not clear what is meant by this.
Several different methods have been tried, and in the end what I did was heat the solution to 80 - 90C, then add 6M HCl until obvious gas evolution
ceased, then allowed the solution to cool. Phenylpyruvic acid precipitates at this point and can be filtered off. There is usually more retained in
solution however, and repeating the heating, acid addition and cooling step will produce more product, although coloured somewhat.
The resulting solid can be recrystalized from 1:1 AcOH:water, but losses are fairly high. The raw product has been used in subsequent experiments
successfully as-is.
When recrystalized, the MP is 174C - 175C, cf lit. 175 - 178C. If impure, I have found MPs ranging from 165C to 173C.
Total yield is 3.56g = 14mmol = 57%, so not excellent, but not so bad as to make it unworthwhile.
___
|
|
|
mr_bovinejony
Hazard to Others
  
Posts: 121
Registered: 20-4-2018
Member Is Offline
Mood: ASS
|
|
Great writeup! But I have to wonder, what is the purpose of this compound?
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
It seems like the benzoic acid substantially complicates the purification. Would starting from acetylglycine instead make it go more smoothly? In your
previous post, you use acetyl chloride so its preparation should be trivial.
http://orgsyn.org/Content/pdfs/procedures/cv2p0001.pdf
http://orgsyn.org/Content/pdfs/procedures/cv2p0519.pdf
Edit: As it seems that the azalactone is readily susceptible to hydrolysis even in boiling water, I strongly suspect the ring-opening with aqueous
acetone in the above link can be disposed of and proceed directly to hydrolysis to the phenylpyruvic acid.
[Edited on 25-1-2021 by UC235]
|
|
|
DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
I have tried the azlactone reaction with acetyl glycine, but the yield of azlactone is much less. With hippuric acid, I regularly get 60% - 70% yield,
but the acetyl glycine only gives 30% to 40% yield (in my hands, at least).
What I haven't done though is calculate the total yield of pyruvic acid from the source aldehyde.
I have also contemplated whether another glycine eg propanamidoacetic acid (propanoic anhydride derivative vs acetic anhydride derivative) might give
better yields, but haven't attempted anything along those lines.
BTW, since those earlier azlactone experiments, I have found that other anhydrides work for the cyclization as well eg benzoic anhydride and benzoic
acetic anhydrides. Polyphosphoric acid can also be used (but haven't tried that either)
And to @mr_bovinejony, I'll publish some experiments using the pyruvic acid in due course.
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Transamination would be handy. Biological systems do it all of the time, but I haven't seen examples, in the chem lab. Am I not looking hard enough?
Sure would be nice if a reaction analogous to the MPV reduction could be utilized.
Reflux the Phenyl Pyruvic acid with Alanine, distill off Pyruvic acid.... Leaving the Phenylalanine as the product.
Of course, if you started with the aniline, you might be able to diazotize it as the hydrobromide, couple with acrylic acid, and react the produced
halo-acid either directly with ammonia, or with Potassium Phthalimide....
Yielding the amino acid. I have a reference somewhere. But, it might be futile. So many ideas; so few of them work. I'll find that reference.....
Wow.... That took a while.
OK. http://www.orgsyn.org/demo.aspx?prep=cv6p0021
[Edited on 26-1-2021 by zed]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Would sodium acrylate react the same way that acrylic acid or would there be a difference in their behavior?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Online
Mood: Big
|
|
Reactions of this form have been reported also with hydantoin, although the hydrolysis product, instead of the arylpyruvic acid, is
the toluene! For some reason, when using hydantoin, hydrolysis removes a unit of oxalic acid.
I think all of these reactions are interesting and hydantoin is easy to make: urea + glycine, urea + glyoxal, etc.
Attachment: henze1940.pdf (408kB)
This file has been downloaded 310 times
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. Seems to me you would be reacting Na with Br, and leaving behind a diazonium acrylate.
Don't know if you can do that. Don't know if you can do that safely.
Me, I'd attempt that coupling, pretty much the way the authors did it.
Never really occurred to me there was such a thing as Sodium Acrylate.
Gonna look that up.....
OK. I'm thinking; no way Jose'.
https://en.wikipedia.org/wiki/Sodium_polyacrylate
Don't know what would happen. Can you get Acrylic acid out of Sodium Polyacrylate? I don't know.
Methyl Methacrylate can be produced by pyrolysing Lucite.
[Edited on 26-1-2021 by zed]
[Edited on 26-1-2021 by zed]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
That was poorly phrased, I mean in the reaction of a radical (in that case the phenyl radical) with an alkene like acrylic acid/acrylate, would there
be any difference in reactivity between acrylate and acrylic acid. Also, how would acrylate/acrylic acid compare in a michael-type addition?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Online
Mood: Big
|
|
Quote: Originally posted by njl  | | Would sodium acrylate react the same way that acrylic acid or would there be a difference in their behavior? |
Because the carboxylate is negatively charged, it affects the dipole moment of the alkene. See also phenolate being more reactive (at CH) than phenol,
catecholate discoloring in air, etc.
The Meerwein arylation is a radical reaction so it's difficult to predict the process but a change is definitely possible: inverse regioselectivity,
formation of an ester, rearrangements, etc. In particular, since radical decarboxylation is a thing, I think you'd see that.
By contrast, the Michael addition is easy to predict: it won't work with a carboxylate. The carboxylate is negatively charged, so it is no longer
electron withdrawing.
[Edited on 26-1-2021 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Now, from my perspective; the question is: "Can I get Acrylic Acid, out of Sodium PolyAcrylate."
That would make Acrylic Acid OTC.
Which would be nice. I can buy Sodium PolyAcrylate online.... Cheap!
So... Anybody got a procedure for Neutralizing Sodium PolyAcrylate, and recovering the free Acrylic Acid?
The interesting thing here, is about synthesizing a PhenylPyruvic Acid, or an Amino Acid. The target molecules.
The idiosyncratic behavior of diazonium salts is not easily understood. And frankly, I'm no expert.
Now, 3,4,5-Trimethoxyphenylalanine, and its decarboxylation product, those are understandable objectives.
[Edited on 27-1-2021 by zed]
[Edited on 27-1-2021 by zed]
|
|
|
DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
OK, not sure I followed all of what was being talked about in the last few posts. But if it is the amino acid you are after, I think you are
over-thinking things.
Some notes below. I'll get around to writing up some of my experiments in this area Real Soon Now.
Firstly, Erlenmeyer derived amino acids from azlactones way back in 1893. A procedure derived from this can be found in (for example) "Note on the
Erlenmeyer Amino-Acid Synthesis" (Harington, McCartney, 1927, doi: 10.1042/bj0210852)
Although high yielding, it uses red phosphorus, hydroiodic acid and acetic anhydride. Pretty much the trifecta of NOT over-the-counter.
The phenylpyruvic acid route is much more approachable. A good reference for synthesis & derivatives is "Synthesis and Properties of the
alpha-Keto acids" (Cooper, Ginos, Meister, doi 10.1021/cr00055a004)
The paper by Baker and Robinson I referenced in my earlier post (doi 10.1039/JR9290000152) describes the creation of an oxime from the pyruvic acid.
Basically, treat the pyruvic acid with hydroxylamine hydrochloride in aqueous sodium hydroxide solution.
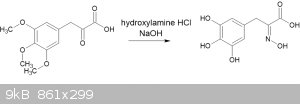
I have performed this experiment with good yield of a white powder with a MP of 166-168C - but I have not been able to find a literature source to
compare against.
I have also attempted the reaction given in "Zinc/ammonium formate: a new facile system for rapid and selective reduction of oximes to amines"
(Abiraj, Gowda, 2003, doi 10.3184/030823403103174281)
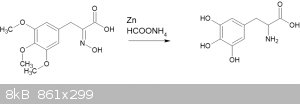
*Something* happened when I attempted this, but I haven't characterized the results at all. Nor did I test for amino acids. Following this up is on
the To Do list.
Much more interesting is the reaction of the pyruvic acid directly with hydroxylamine hydrochloride WITHOUT going to the oxime. Refer "The conversion
of alpha-keto acids and of alpha-keto acid oximes to nitriles in aqueous solution" (Ahmad, Spenser, 1961, doi 10.1139/v61-169)
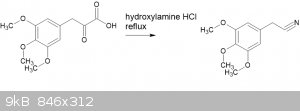
I have had modest success with this. Not excellent yields, but plenty enough to verify MP.
I will leave it to others to take the reaction beyond this point 
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Why do most of your pictures except the last include a triple O-demethylation?
I guess you must have forgotten to attach the O-methyl in chemdraw or whatever you're using, and copied the triphenol prematurely instead of the
trimethyl ether 
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Lots of references. I like that. Thank you.
Anybody got a procedure for converting Sodium PolyAcrylate to Acrylic Acid?
|
|
|
DrDevice
Hazard to Self
 
Posts: 74
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
Sigh - no, there isn't any demethylation. Copy & paste error. Thanks for pointing it out.
|
|
|