Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Report: Gallium (iii) iodide & how to dissolve gallium fast
I have tried on and off over a period to react gallium with iodide to make GaI3. I tried to react gallium metal and iodine in range of solvents in
various temperatures (DCM, toluene, xylene) and although there was also some product formed the process was very slow, it would take days to react a
few gram of gallium. Plus I ended up with a brown solution that did not want to give up any solid compound (same as lower down...).
The last two days I tried something else. What I stumbled upon is a way to get gallium metal into solution relatively quickly and easily and I also
seemed to have made some GaI3 but I do not quite understand the reactions that takes place and I hope members can help with this. This is the process:
- 2g gallium metal in an evaporating dish - size as per photo below.

- Add 12ml "concentrated" hydrochloric acid - this is what I buy here as pool acid, says minimum 330g/L, fumes, and is quite clear.
- Weight out 10g iodine crystals. Going by the simple equation
2Ga + 3I2 = 2GaI3, 2g gallium will react with around 11g iodine, and the reaction proceeds accordingly as I observed during a few runs: only once 11g
of I2 is exceeded does the solution remain dark. 10g is a safe limit to ensure all I2 is consumed.
- Start adding the I2 in small amounts, for example 1 gram at a time. With the first addition it will initially appear as if not much is happening but
then the reaction suddenly takes off.
- With each addition, wait and enjoy the gallium ball running around 'chasing' the iodine. No external heat required (but my shed is at around 30C),
but the solution does heat up a bit. I hope the below video works.
Attachment: 2.MOV (2.1MB)
This file has been downloaded 266 times
- After each addition wait until the solution is crystal clear before adding the next. Towards the end it may be neccessary to stir with a glass rod
to get is clear. Finally, after adding 10g I2, there is only a tiny ball of Ga left and the solution is still water clear! The remaining ball of
gallium was extracted with a pipette.

- I tried heating the evaporating dish on a steam bath for many hours. The weight of the contents reduced from some 24g to 13g and it turned pale
brown but then did not change any more. So in future I will place it direct in the sand bath.
- On the sand bath for 90 minutes. Sand temperature close to 300C. Acrid smoke coming off, but no iodine fumes. After 90 minutes what was left was a
soft brown paste:

- Once cooled the paste seemed dry and hard. Yield was 6g.

- The product dissolved easily in water, and when lead nitrate solution is added yellow lead iodide precipitates.

So I assume the product is indeed Gallium Iodide. It seems quite stable, as mentioned above prolonged heating at near 300C did not yield any iodine
smoke.
But, there should have been some 12g GaI3. Not just 6g. Where is the rest of the iodine and gallium? I am quite sure some of the I2 escaped as HI,
thus the weight loss and acid smoke on the sand bath. For sure some GaCl3 would have formed as well, and I suspect that this could have sublimated as
300C. Any thoughts / idea will be welcome!
If one only wants to get gallium in solution fast to make other gallium compounds, then, after dissolving the last of the I2 and having the clear
solution, one could possible neutralise the solution with NaOH and ppt out the gallium as oxide/hydroxide which can then be used as a basis for
following reactions? I will try this at some stage.
I also saw during earlier runs (with solvents) that adding just excess water causes a white ppt to form, or that adding Na2S yields a white ppt that
in turns easily dissolves in acids (and gives lovely H2S) so presumably that white ppt was gallium sulphide.
Still so much to be investigated further.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Noce work. Is the acid hot?
[Edited on 4-2-2021 by vano]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I think you lost no gallium, but quite some iodine. Most likely your product hydrolyzed and you have a mixed oxide/iodide, something with approximate
formula GaIO. This certainly can explain that you only have 6 grams of solid and at the same time that no gallium is lost.
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Vano - nothing heated beforehand, acid at my room temp (around 30C). But it heats up as the reaction progresses, in fact if too big portions of iodine
is added to quickly in succession it starts to expel very small bits of iodine.
Woelen - thanks. The iodine loss would have been as HI fumes? No purple iodine vapour was observed at any time.
|
|
|
violet sin
International Hazard
    
Posts: 1475
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
THE PREPARATION OF GALLIUM TRIBROMIDE AND GALLIUM TRIIODIDE*
BY WARREN C. JOHNSON AND JAMES B. PARSONS
Attachment: j150312a007.pdf (299kB)
This file has been downloaded 243 times
"Equivalent quantities of these two elements were reacted in a small Pyres tube in the absence of air and moisture. Reaction did not take place at
room temperat~re;'~ however, on warming gently with a flame, a vigorous reaction followed with the evolution of much heat and light."
" Other Properties. Gallium triiodide sublimes readily in vacuo at a temperature as low as 160". It hydrolyzes readily and is hygroscopic. When it is
exposed to the air iodine fumes are liberated and the residue appears to be much darker than the original salt. At higher temperatures decomposition
takes place."
==≠===≠======÷=======≠=====≠===÷÷÷======
The salts were prepared in an interesting manner with a crazy glass setup. Decent read by my opinion. But they used some high vacuum and glass
blowing skills with numerous closures on a glass tree shaped thing, lol. So out of my capabilities. Didn't seem like something a guy would
necessarily want to try.
But they had some chemical properties and such listed, it's 5 pages, hope it helps
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi Violet Sin the document is an interesting and useful read, thank you! With what I have in terms of knowledge and equipment I am not sure if I can
ever know exactly what the 6 gram of product is but as Woelen says in his post above GaIO explains both the 2g gallium started with as well as 6g of
product.
The appeal of the reaction is that it offers a way to the home amateur to put gallium in solution quite fast. My next step is to see if I can get 3.5g
gallium hydroxide using this method, starting with 2g gallium metal.
Another interesting side observation is that there is some green color present in the crucible from an earlier run when I was still trying different
things. I'm not sure if this hints that some Gallium (i) product was formed; GaI is said to be green. Still to be investigated further 
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Quote: Originally posted by Lion850  | | Woelen - thanks. The iodine loss would have been as HI fumes? No purple iodine vapour was observed at any time. |
Yes, it would be in the form of HI fumes. It's as with AlCl3. This compound cannot be prepared from an aqueous solution of AlCl3. If
you heat such a solution, then when it is very concentrated, you get loss of HCl and oxide (or hydroxide) remains behind. The final product will be a
mixed chloride/oxide/hydroxide of indeterminate stoichiometry, something close to AlOCl. Many easily hydrolyzable compounds have this property. The
same is true for iron(III), bismuth(III), antimony(III), and quite a few others.
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Attempt at Gallium Hydroxide using the above method to quickly get Gallium in solution:
2.2g gallium metal was added to a evaporating dish, then 12ml concentrated HCl added, then a total of 10.2g iodine added in small portions, as
described in more detail in the first post. So in less than 10 minutes I had my gallium in solution. As before, the result after all the iodine
reacted was a crystal clear solution (I did notice something I did not see earlier: when the last few portions of iodine was added, the iodine starts
reacting with the solution with tiny bubbles even before the ball of gallium gets close. I wonder what this reaction is?).
The next phase now was to try and get the gallium out of solution as gallium hydroxide. And as a secondary reaction, to get the iodine out as a useful
iodide salt.
Wikipedia says gallium hydroxide forms as a gel when ammonia is added to a solution of a gallium salt. So I started to add 28% ammonia solution to the
evaporating dish drop by drop in 2 gram portions. After each 2 grams the contents was stirred with a glass rod and the pH checked using indicator
strips.
Initially each drop caused a bit of a jelly like ppt which soon dissolved. As the addition progressed it took longer and longer for the ppt to
dissolve. After a total of 14.5g of ammonia solution was added the pH was still <2, but the addition of another 2g (total 16.5) caused the pH to
raise to around 10. The contents was now also more gel than anything else.
The solution was gravity filtered, and the remainder washed a few times in the funnel with water. This took quite a while. This gave a slightly cloudy
filtrate and 21.7g of white gel as remainder, photo below:

The gel remainder was put straight on a steam bath, while processing of the filtrate continued. I decided to extract the iodine from the filtrate by
conversion to lead iodide PbI2, which should ppt out and thus be easy to recover with minimal losses. Going by the initial amount of I2 being 10.2g,
theoretical yield of PbI2 was 18.6g
14g lead nitrate was dissolved in 40ml water, and mixed with the filtrate. Yellow Lead iodide immediately formed:

The solution was filtered and the yellow remainder dried on the steam bath until a constant weight was reached. 15.3g of PbI2 was recovered. This is
some 82% of the theoretical.

The gel initial remainder, which should have contained the gallium product, took many hours to reach a steady weight. After some 6 hours on the steam
bath the final weight was 7.5g, but the color was now white mixed with brown as shown below:

The maximum expected Ga(OH)3 was only 3.2g and it should be white. I read that gallium hydroxide gels are quite resistant to drying. The color also
made me think that some ammonium iodide may have been mixed in, which will also explain why my lead iodide recovered was missing a few grams.
I read that ammonium iodide was quite soluble in methanol; and assumed / hoped that gallium hydroxide would not be. I fast-stirred the product with
150ml of boiling methanol for 30 minutes. This gave a pale yellow supernatant liquid with a heavy white ppt. Photo below.

The supernatant liquid was decanted through a filter, more methanol added for another wash, decanted through the same filter, and the white ppt was
then transferred to the same filter and rinsed with yet more methanol. Unfortunately there was some mechanical losses as the fine white product was
stuck to all sides of the beaker; such losses inevitably have a bigger effect on recovery % when working with small quantities in big-ish beakers.
The while salt was dried on a SB (which was easy as the methanol evporates fast) and the final recovery was 2.3g of dry white powder. This is a 71%
yield (assuming Ga(OH)3.)

The product, which presumably is Ga(OH)3, dissolves fast in dilute HCl - I know this from having cleaned the evaporating dish and implements with
dilute acid. Once the HCl dissolved the tiny bit of leftover salt on the dish I added a few drops of potassium dichromate solution to see if a get a
gallium chromate ppt - a dark-ish ppt formed but quickly dissolved again. I'm not even sure if gallium forms a chromate or dichromate; cant find
anything on google.
Does anyone know of any colorfull gallium salts or double salts (other than green gallium (i) salts which are not so straightforward to make).
[Edited on 13-2-2021 by Lion850]
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
α-Ga2S3 is yellow. This can be made from elements. You cannot precipitate it from a solution of gallium(III) salt, because Ga2S3 hydrolyse in to
Ga(OH)3 and H2S.
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
I was interested to see if this combination of concentrated hydrochloric acid and iodine will dissolved other metals. I tried tin first.
5g of small tin granules were placed in an evaporating dish and 13g concentrated HCl added. Iodine was then added in small portions over the course of
a days. A total of 9g I2 was added. When I next went to the shed after some 4 days this is what I found:

The 'mounds' seemed to have formed around the granules and were by now hollow as the tin was all consumed. I popped the mounds to get this:

Which was then dried on a steam bath until a steady weight was reached by which time there was also no more acid smell. Color paled a bit during the
drying as below.

Total recovery was 13.6g. I either ended up with SnI2 or a mic of SnI4 and SnCl2; either of these more or less explains the final product weight as
far as I can work out.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
I think it is a good method. Tin iodide has beautiful colour. Also from my experience i know that Gallium is hard dissolved metal in acids(I do not
mean that it doesn't dissolve, just it takes long time). For example i made lots of gallium nitrate, because its easy source of gallium cation in
solution.I was doing it for two days and i lost lots of nitric acid(but it's cheap). Very good method, because its fast.
[Edited on 3-3-2021 by vano]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Probably you are successful at dissolving gallium due to the formation of some GaX4- species, where X = Cl, I. IIRC the gallium
trihalides are in fact quite lipophilic in pure form.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Vano - what did you do with the gallium after you made "lots of gallium nitrate"? I have been looking out for colourful gallium compounds to make but
cant really find any. Does gallium chromate exist?
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Ga2S3 have two forms - yellow (which can be made from elements) and white.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
I did not make colored compounds. Basically all white. I made nitrate because I thought I would use it in the future, though once I tried to make
selenide. I do not know why I made so much nitrate, to tell you the truth. The photo in the infobox is some part of my gallium nitrate nonanhydrate:
https://en.wikipedia.org/wiki/Gallium_nitrate
Chromate I have not seen anywhere but it is worth a try, unfortunately now I am in another city, I have gallium nitrate here but no chromate. If I
have seen anything, I will definitely write to you
Bedlasky: you are right. Also every preparation method is hard. I like this reaction, but it also need high temperature, but it's consider one of the
simple reaction.
Ga2O3 [2 Ga(OH)3] + 3 H2S = Ga2S3 + 3H2O [6 H2O]
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Vano: Reacting Ga2O3 with H2S (of course in dry state, Ga2S3 hydrolyses in water) you obtain white sulfide.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
I found very old potassium dichromate crystals. I made solutions of nitrate and dichromate, when i added dichromate in nitrate solution then nothing
precipitated. But next i added potassium hydroxide solution, something precipitated, i dont know it is a hydroxide or chromate, but I don't think this
yellow compound only contain oxide and colour is caused by chromate(i filtered it. But i didn't add extra water to avoid hydrolysis.). I tried to
washed it. But many of them maybe hydrolyzed or it is soluble in chromate solution. But some of them in water have yellow colour.
  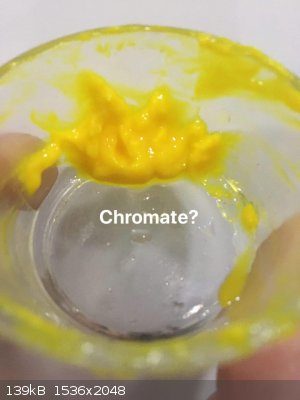
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
I know that this reaction needs 600°C.
[Edited on 4-3-2021 by vano]
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
I washed 3 times this small amount of compound in big test tube. It has yellow colour and i think this water is enough. Solution have yellowish
colour, but i believe this is hydrolyzed chromate and not dilute solution of potassium chromate. Also i just tried to dissolve antimony in iodine and
HCl solution, but crystal looks same.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
This is what it looks like after hours. If this precipitated substance were conventionally potassium chromate, it would dissolve in this amount of
water, while gallium oxide is white, so it may be gallium chromate.

|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi Vano thank you! I'll try a few things but don't know when I will get back to Gallium; now struggling to get a dry chromium b-alanine complex.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Quote: Originally posted by Lion850  | | Hi Vano thank you! I'll try a few things but don't know when I will get back to Gallium; now struggling to get a dry chromium b-alanine complex.
|
Interesting chromium compound. Good luck!
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
I used very small antimony crystal. I just tried and it really works.

[Edited on 7-3-2021 by vano]
|
|
|
Lion850
National Hazard
   
Posts: 514
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi Vano I like the color! What this the result of dissolving antimony in HCl with iodine? I previously made SbI3 by reacting antimony powder and
iodine in hot xylene for a few hours. Color is quite similar to what you got.

[Edited on 7-3-2021 by Lion850]
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
I just tried . I can also say that the reaction is not as fast as in the case of gallium and tin. I will probably make more in the future and let you
know the details. Everyone uses an organic solvent, so I think this method is a good alternative.
[Edited on 8-3-2021 by vano]
|
|
|