| Pages:
1
2 |
pip
Hazard to Others
  
Posts: 109
Registered: 19-9-2008
Member Is Offline
Mood: No Mood
|
|
Enough is enough, this guy needs to take his mental health issues out in someone else.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
If you actually knew what you were doing, I wouldn't be so critical. It's pretty clear that you can neither respond to my arguments nor use ketene
safely. Having revealed that to everyone, I've accomplished something.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
pip
Hazard to Others
  
Posts: 109
Registered: 19-9-2008
Member Is Offline
Mood: No Mood
|
|
Only thing you’ve accomplished is proving that you argue just to argue, you haven’t contributed anything of substance to the conversation.
Repeating that zinc acetate isn’t a good method isn’t anything new. Posting synthesis methods already known isn’t contributing. The only thing
you’ve successfully done is derail the conversation. Even when I tried to put it back on track by agreeing that I wouldn’t do the zinc method
because I prefer ketene, but I do support the OP experimenting with zinc under different experimental conditions you choose to pick a new argument
about the method I personally prefer as if that somehow affects you personally.
Can we get back to the topic now? Is that ok with you?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
You realize it was me who read through the 200-page PhD thesis you posted and highlighted the yields for everyone, correct?
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
pip
Hazard to Others
  
Posts: 109
Registered: 19-9-2008
Member Is Offline
Mood: No Mood
|
|
Are you done yet?
Let the guy experiment, no one needs your blessing
[Edited on 9-2-2021 by pip]
|
|
|
ChemichaelRXN
Hazard to Others
  
Posts: 103
Registered: 7-10-2010
Member Is Offline
Mood: Universal Eye
|
|
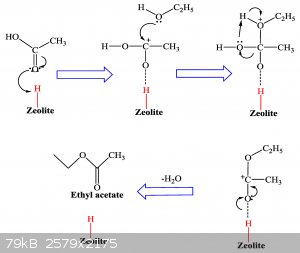
I dont need acetic anhydride, but I was thinking, do you think reacting 2 moles of acetic acid with the catalyst H-beta-zeolite would form the acetic
anhydride? I have seen a number of reactions where diols are joined, but maybe steric hindrance from the ketone group of carboxylic acid would prevent
it. Not sure...
There are a number of things you can do with h-beta-zeolite.
Esters. https://www.mdpi.com/2073-4344/9/9/758/htm
Amides. http://nopr.niscair.res.in/bitstream/123456789/40904/1/IJCA%...
Cyclization joining diols. https://pubs.rsc.org/en/content/articlelanding/2018/gc/c7gc0...
Very versatile. If anyone want some for free i have about 25 grams x 3 of h-beta-zeolite I will give away, just PM me!
[Edited on 10-2-2021 by ChemichaelRXN]
You are the same perception looking out, from the same elements around the universe.
You are everything to be anything to begin with.
Https://you-are.space
Https://syntharise.com
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
acetic anhydride is prepared by the reaction of ketene (ethenone) with acetic acid at 45–55 °C and low pressure (0.05–0.2 bar).
I hope the paper that states that zinc acetate is not referring to acetone first produced (as an intermediate) then cracked into acetic anhydride
Simular to calcium acetate gives off acetone when heated too.
It maybe possible that it is generated directly. Has anyone attempted to use other transition metal acetates.
If acetic acid can just be dessicated under vacuum using calcium chloride, magnesium sulfate, sulfuric acid or phosphorous pentoxide.
Here is from prep chem
acetic anhydride was prepared by placing 20 grams of dry acetic acid in a flask, fitted to a condenser and then dropping 20 grams of oxalyl chloride
in the acetic acid. The water was removed from the acid by freezing it in a freezing mixture until there was just a little liquid left in the center.
Wow apperently Acetic anhydride can be used as a dehydrating agent for the formation of anhydrides.
Acetic anhydride can also by obtained from an anhydrous sodium acetate and phosphorus oxychloride. From practical point of view it is not necessary
for the preparation of acetic anhydride isolate the acetyl chloride; it is better to allow acetyl chloride to act directly on an excess of the
anhydrous sodium acetate, so that from the phosphorus oxychloride and anhydrous sodium acetate the acetic anhydride is directly obtained
Here is silver acetate
A solution of silver acetate in pyridine absorbs hydrogen, producing metallic silver and anhydrous acetic acid
2 CH3CO2Ag + H2 → 2 Ag + 2 CH3CO2H
[Edited on 10-2-2021 by symboom]
|
|
|
pip
Hazard to Others
  
Posts: 109
Registered: 19-9-2008
Member Is Offline
Mood: No Mood
|
|
Phosphorus pentoxide does form acetic anhydride in acetic acid. But it’s not the easiest chemical to get a hold of. Some of us can, some of us
can’t. That’s why ketene is still the preferred method for a lot of people. It’s downsides are outweighed by its simplicity. There’s a lot of
YouTube ketene videos that cover it well. Including the fact that while ketene and water will make acetic anhydride, you have to stop at 70% acetic
acid, distill the acid to remove the water, then continue. For some reason polymerization becomes a problem past 70% acetic acid without first
removing the water.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2691
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Quote: Originally posted by symboom  | | acetic anhydride is prepared by the reaction of ketene (ethenone) with acetic acid at 45–55 °C and low pressure (0.05–0.2 bar).
|
Come on, you know better than this.
Quote: Originally posted by ChemichaelRXN  | | I dont need acetic anhydride, but I was thinking, do you think reacting 2 moles of acetic acid with the catalyst H-beta-zeolite would form the acetic
anhydride? I have seen a number of reactions where diols are joined, but maybe steric hindrance from the ketone group of carboxylic acid would prevent
it. Not sure... |
There's a lovely thread about acetic anhydride in general. It's stickied.
Quote: Originally posted by pip  | | That’s why ketene is still the preferred method for a lot of people. It’s downsides are outweighed by its simplicity. |
No, ketene is a dangerous, stupid idea that nobody should be encouraged to pursue. I thought I explained this to you. There's one video from
Doug's Lab and even his fans pointed out he was being reckless.
Chlorine, bromine, hydrogen chloride and other dangerous gases used for this become irritating long before they become toxic. Ketene can become toxic
before it becomes irritating. There's a reason nobody encourages it here.
Also, plenty of people failed at using phosphorus pentoxide in the Ac2O sticky. It's easy to end up with "charred black crap". You would know this if
you bothered to do your homework.
[Edited on 10-2-2021 by clearly_not_atara]
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
pip
Hazard to Others
  
Posts: 109
Registered: 19-9-2008
Member Is Offline
Mood: No Mood
|
|
What part of no one cares what you have to say are you not getting? Stop with the personal mission of discouraging others from doing something because
you can’t do it safely. How many more posts are you going to make regurgitating the same crap? You’re against it, we get it, now move on.
I guess this paper is fake then...
https://www.ijraset.com/fileserve.php?FID=24897
[Edited on 10-2-2021 by pip]
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
You're the one in the wrong here, pip. Atara is spot on about the risks of the ketene procedure that you are being so blasé about. It's not
"preferred for a lot of people." You can probably count the number of amateurs who actually use it on one hand. Who knows, maybe you're one of them.
You need a really good fume hood. Not just a box with a fan blowing out of it. You need to be certain that all of the air inside of it is circulated.
You need a purpose-built apparatus. Not some cobbled together glassware that could pop open at any moment. And finally, you need experience working
with other gases that are less dangerous so that you realize just how easy it is for traces of gas to escape even the best of hoods. Maybe you have
all that, pip, maybe you don't. I don't care whether you do it or not. The problem is how you casually suggest it as if it's something that everyone
should be doing.
As for this thread, zinc acetate is clearly a dead end. Other methods for acetic anhydride can be discussed in the sticky.
|
|
|
Texium
|
Thread Closed
10-2-2021 at 14:18 |
| Pages:
1
2 |