ChemichaelRXN
Hazard to Others
  
Posts: 103
Registered: 7-10-2010
Member Is Offline
Mood: Universal Eye
|
|
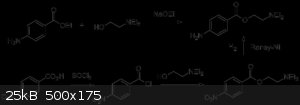
Benzoyl chloride + 4-Aminoadamantan-1-ol
Hello,
I am going to be producing an esterification product with Benzoyl chloride + 4-Aminoadamantan-1-ol. 4-amino-1-adamantanyl benzoate. For this reaction
that takes off an HCl to join the two compounds, I am guessing no catalyst is required like H2SO4.
I am looking forward to obtaining this final product as I havent seen it documented anywhere. It would make a great compound to research and I am
guessing affinity would be to NMDA as an agonist and also Dopamine as and agonist and who knows what else.
If everything goes as planned, I will add to this thread with the synthesis.
It is the same way procaine is synthesized as shown.
You are the same perception looking out, from the same elements around the universe.
You are everything to be anything to begin with.
Https://you-are.space
Https://syntharise.com
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
The amine shown in the procaine synthesis is tertiary so it can't be acylated by benzoyl chloride, 4-Aminoadamantan-1-ol is a primary amine and will
therefore react with benzoyl chloride. You need to protect the amine (at first glance phthaloyl group seems like it would be convenient but I couldn't
say for sure).
|
|
|
ChemichaelRXN
Hazard to Others
  
Posts: 103
Registered: 7-10-2010
Member Is Offline
Mood: Universal Eye
|
|
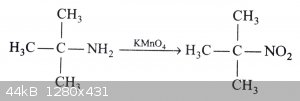
Ok, interesting. Overlooked that, thanks. Maybe I can oxidize the amine to a nitro and reduce after or add a methyl group. That seems the most
feasible based on chemicals available. I have KMnO4...it might work.
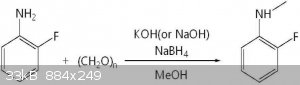
To add a methyl:
First, 120 mg (4 mmol) of paraformaldehyde was added to 25 mL of methanol under normal temperature and closed conditions, and the mixture was refluxed
at 85 캜 until paraformaldehyde was dissolved. Subsequently, 296 mg (2.67 mmol) of 2-fluoroaniline and 299 mg (5.34 mmol) of potassium hydroxide were
dissolved in 3 ml of methanol at room temperature, followed by mixing with paraformaldehyde reactant dissolved in methanol, Lt; / RTI > After 2
hours, the solution was cooled to 0 ° C and then 202 mg (5.34 mmol) of sodium borohydride was added slowly and refluxed again at 85 ° C for 1 hour.
When the reaction was completed, the methanol was removed by a vacuum concentrator, and then 25 ml of water and 20 ml of ethyl acetate (3 times
repeated) were added to take only the organic layer. The organic layer was washed with 20 ml of water and 20 ml of saturated brine, dried over
magnesium sulfate and concentrated in vacuo to give 2-fluoro-N-methylaniline (yield 95%, purity 99%) in the form of a pink oil Example 1 was obtained.
[Edited on 14-2-2021 by ChemichaelRXN]
You are the same perception looking out, from the same elements around the universe.
You are everything to be anything to begin with.
Https://you-are.space
Https://syntharise.com
|
|
|
ChemichaelRXN
Hazard to Others
  
Posts: 103
Registered: 7-10-2010
Member Is Offline
Mood: Universal Eye
|
|
I guess the sodium ethoxide pathway would work. I cant find too much on this one though. I will make ethyl benzoate and go for it.
You are the same perception looking out, from the same elements around the universe.
You are everything to be anything to begin with.
Https://you-are.space
Https://syntharise.com
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Maybe chlorinate with HCl to get 4-Aminoadamantan-1-chloride, followed by reaction with sodium benzoate
|
|
|
ChemichaelRXN
Hazard to Others
  
Posts: 103
Registered: 7-10-2010
Member Is Offline
Mood: Universal Eye
|
|
That sounds like a good idea. Any example of this reaction/procedure?
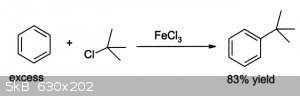
That is a pretty useful compound because I guess I can also try reacting the 4-amino-1-chloroadamantane with benzene to attach them as well, with an
HCl byproduct and using FeCl3 catalyst. These new research compounds would be very interesting to add to my collection. Definitely unexplored.
[Edited on 15-2-2021 by ChemichaelRXN]
You are the same perception looking out, from the same elements around the universe.
You are everything to be anything to begin with.
Https://you-are.space
Https://syntharise.com
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
No specific ref, but I think that once the amine salt is formed the alcohol can be halogenated a la t-butyl alcohol to t-butyl chloride.
http://www.rsc.org/suppdata/books/184973/9781849739634/bk978...
|
|
|