chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Drying pseudonitrosites
Ref:
https://erowid.org/archive/rhodium/chemistry/pseudonitrosite...
In this paper, it is emphasized that pseudonitrosite dimer be vacuum dried and then air dried before the reaction with alcoholic KOH. Yet it is stated
that the pseudonitrosite decomposes quickly upon storage producing HCN and Nitrogen oxides. vacuum filtration is not available to me right now, and
I’m unsure if air drying is even safe due to the short decomposition time.
Must the reaction between the pseudonitrosite dimer and alcoholic KOH be anhydrous? if so, what is the maximum amount of time you could safely allow
the pseudonitrosite to air dry?
|
|
|
dawt
Hazard to Self
 
Posts: 74
Registered: 9-5-2016
Location: EU
Member Is Offline
Mood: fluorescent
|
|
Doesn't need to be anhydrous. Leaving the pump running for a minute or two after the filtration works well, but if you don't have access to one you
might try putting your filter on a paper towel after filtration to get rid of more moisture?
At room temperature the pseudonitrosites visibly begin discolouring after a few minutes. If you're left with a fine precipitate this might cause you
some headaches if the gravity filtration takes too long.
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Thanks so much, this is great info. do you have any references, or are you speaking from experience?
|
|
|
dawt
Hazard to Self
 
Posts: 74
Registered: 9-5-2016
Location: EU
Member Is Offline
Mood: fluorescent
|
|
I messed around with eugenol many years ago, making all sorts of derivatives to see what they smelled like. Made pseudonitrosites two or three times.
If I remember correctly the yield was greatly improved by keeping the reaction mixture in an ice bath during the gassing with N2O3, and starting the
workup immediately after the color change. You'll see what I mean when you run the reaction. 
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Ah, so the dimerization of the pseudonitrosite is quite rapid then? I assume it clears up within a few minutes after the initial change. No wonder why
they got such low yeilds in my reference; they gave it hours to waste away, and probably didnt keep it cool enough for the maximum amount of N2O3 to
stay in solution.
Thank you so much, this has been of great help! (:
|
|
|
dawt
Hazard to Self
 
Posts: 74
Registered: 9-5-2016
Location: EU
Member Is Offline
Mood: fluorescent
|
|
IIRC the gassing took a few hours... Memory's a bit hazy since it was so long ago. Pretty sure I generated the N2O3 in situ at first and wasn't happy
with the results, so I set up an external N2O3 generator on my second attempt and made a few more changes, as described above.
Try purging your apparatus of oxygen before making the N2O3, or you'll get a bunch of brown NO2... Instantly and permanently discolored my PVC tubing.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Think about it this way: what do you expect to be harder to dry, the pseudonitrosidte or the KOH? Because once they're in the same phase it's all the
same. Anhydrous KOH is way harder than some oily organic compound.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
dicyanin
Hazard to Self
 
Posts: 57
Registered: 29-3-2020
Location: Europe
Member Is Offline
Mood: inquisitive
|
|
It never was an issue for me as the pseudonitrosite is washed a few times with alcohol and finally with ether.
sic transit gloria mundi
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
"Must the reaction between the pseudonitrosite dimer and alcoholic KOH be anhydrous?"
Interesting question. Technically, Alcoholic KOH cannot be anhydrous. There is an equilibrium wherein KOH+Alcohol<--->K-Alkoxide+H2O. Alcohol
can be anhydrous. Potassium Hydroxide can be anhydrous. Potassium Ethoxide can be anhydrous. But, Alcohol plus KOH... No. Just part of the deal.
[Edited on 12-5-2021 by zed]
[Edited on 12-5-2021 by zed]
|
|
|
dicyanin
Hazard to Self
 
Posts: 57
Registered: 29-3-2020
Location: Europe
Member Is Offline
Mood: inquisitive
|
|
Pondering over the stability of pseudonitrosites, I remembered I still had a sample of isodillapiole in my collection, so I decided to do an
experiment.
Preparation of isodillapiole pseudonitrosite
A solution of 20.7 g (0.3 mol) sodium nitrite in 35 ml distilled water was prepared and added to a 500 ml round-bottom flask with stirbar. A solution
of 6.6 g (0.03 mol) isodillapiole [1] in 50 ml diethyl ether was added, the flask fitted with a Claisen adapter, an addition funnel, and an outlet to
vent the noxious fumes outside. The flask was placed in an ice/water bath (with plenty of ice) on a magnetic stirrer. In the addition funnel was
placed 75 g 20% sulfuric acid. The acid was added dropwise to the stirring two-phase mixture over a period of 3 hours. (using the principle "when you
see brown NO2 fumes you're adding the acid too fast.")
At about 1/5th of the acid added, a cloudiness was observed. This then turned into a thick cream-coloured precipitate towards the end of the addition.
It was left stirring in the ice/water bath an additional hour. Air was pumped in through the addition funnel using an aquarium air-pump to remove as
much nitric oxide fumes as possible from the system before opening it.
The mixture was filtered using gravity filtration through 2 coffee-filters (obviously this should be done in a well-ventilated area). The obtained
solids were washed with distilled water, 4x with methanol and finally with a good portion of diethyl ether. When gravity filtration is done, the
filter is pressed by hand against the funnel as to remove as much solvent as possible. Then it was air dried for 1 hour. Tan coloured crystals of
isodillapiole pseudonitrosite were obtained.
They weighed 15.3 grams after air drying for over an hour. My impression is that the solids seem to absorb some of the ether. It is not easy to get
them to completely dry like this. I took a 0.3 gram sample of the pseudonitrosite in a capped sample contained to observe the decomposition rate. As I
am typing this, the sample is still white and crystalline after 48 hours, no brown NO2 fumes can be seen, there are still no signs of decomposition.
The reason why I did this experiment was in the first case to observe the stability of pseudonitrosites at first hand. At least for isodillapiole, it
is much more stable than often assumed.
[1] The isodillapiole used in this experiment was prepared about six months ago. It was obtained by refluxing dillapiole in 4M ethanolic KOH for 15
hours. The product was isolated following common procedure and recrystallised twice from petroleum ether. A yellowish-white crystalline product was
obtained that darkened somewhat on standing. The dillapiole was obtained from fractionation of Indian dill seed oil (Anethum sowa), it is the main
fraction after removal of carvone.
     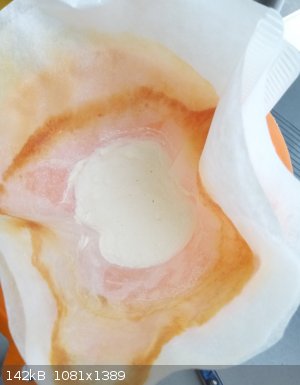   
sic transit gloria mundi
|
|
|
dicyanin
Hazard to Self
 
Posts: 57
Registered: 29-3-2020
Location: Europe
Member Is Offline
Mood: inquisitive
|
|
Preparation of beta-nitroisodillapiole
The above pseudonitrosite was added in small portions to a strongly stirred chilled solution of 50 ml 8% ethanolic KOH [2], placed in an ice/water
bath. The temperature was 2-3°C during addition. Only minimal foaming was noted, after addition the mixture was stirred for an additional 30 minutes.
It was then filtered, again using gravity filtration (through a single coffee-filter), and the solids washed with a portion of ethanol, added to the
filtrate. [3]
The filtrate was poured on 30 grams of crushed ice, and the mixture was carefully acidified by adding 50 ml 5% aq. HCl in small portions with
stirring, so that the temperature was never allowed to rise above 10°C. A fine, canary-yellow precipitate was obtained, which was isolated again by
gravity filtration (through 2 coffee-filters). The precipitate was washed with ice-cold water, and the washing added to the filtrate which made it
turn cloudy. After standing for 24 hours a (small) second crop is obtained from this as yellow needles. The first crop weighed 2.2 g and the second
0.4 g so the total yield of beta-nitroisodillapiole was 2.6 g (32% based on isodillapiole).
[2] The ethanol used was technical grade 95% denatured ethanol, sold as "bio alcohol fuel". The denaturant agent is unlisted, but on dissolving KOH
the (initially clear) solution turned an orange colour.
[3] These beige coloured solids were suspended in boiling ethanol, filtered again, and dried and saved. As such 9.0 grams of potassium hyponitrite
(K2N2O2) were obtained as a white solid. This is in itself an interesting compound, it can be used as reducing agent
and the corresponding acid is a tautomer of nitramide.
In previous experiments (long ago I admit) I was never able to obtain the higher yields mentioned in the pseudonitrosite FAQ. Results were always in
the range of the above using other substrates too.
    
sic transit gloria mundi
|
|
|
dicyanin
Hazard to Self
 
Posts: 57
Registered: 29-3-2020
Location: Europe
Member Is Offline
Mood: inquisitive
|
|
This is the sample of isodillapiole pseudonitrosite I kept in a capped container. It is now 2 days and 2 nights after the experiment and I still can't
see any clear signs of decomposition.
 
sic transit gloria mundi
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Nice work dicyanin! I must admit that I have never had need to prepare pseudonitrosites but the ability to recovery the hyponitrite is intriguing.
|
|
|
dicyanin
Hazard to Self
 
Posts: 57
Registered: 29-3-2020
Location: Europe
Member Is Offline
Mood: inquisitive
|
|
Thank you. I've always found it a very elegant reaction. And I'm more interested in the chemistry muse, than in the product obtained by taking it a
step further. My capped sample of isodillapiole pseudonitrosite still shows no visible signs of decomposition, it is still white and no sign of brown
NO2 fumes when holding it against a white background. I wonder if all pseudonitrosites are more stable than we assumed, or if it is just
this particular substrate?
Maybe it would it an idea to use paper indicators to measure decomposition?
The hyponitrite has some interesting points. It can reduce iodine, and the corresponding acid apparently has energetic properties. It must be prepared
from silver(I)hyponitrite in strictly anhydrous conditions though. Merely acidifying K2N2O2 will result in generation
of N2O, laughing gas.
https://en.wikipedia.org/wiki/Hyponitrous_acid
| Quote: | | In aqueous solution, it is a weak acid (pKa1 = 7.21, pKa2 = 11.54), and decomposes to nitrous oxide and water with a half life
of 16 days at 25 °C at pH 1–3 |
Alkyl hyponitrites can also be prepared from silver(I)hyponitrite and alkyl halides. Some of them have energetic properties, methyl hyponitrite can
only be handled in solution. According to J. Org. Chem. 48 (1983) 3728-33 (doi:10.1021/jo00169a023) they are a convenient source of alkoxyl radicals.

[Edited on 14-5-2021 by dicyanin]
sic transit gloria mundi
|
|
|