Illegal Parkinson
Hazard to Self
 
Posts: 75
Registered: 2-10-2005
Member Is Offline
Mood: No Mood
|
|
N-Boc-7-Azanorborn-2-one [152533-47-6]
Does anybody know if N-Boc-pyrrole can react with ketene in a single step to give the title compound?
I think actually you have to catalytically hydrogenate the olefin bond.
I know in the lab it was made using a different procedure, then it becomes like a slave labour than trivially easy to do.
[Edited on 21-3-2021 by Illegal Parkinson]
|
|
|
Illegal Parkinson
Hazard to Self
 
Posts: 75
Registered: 2-10-2005
Member Is Offline
Mood: No Mood
|
|
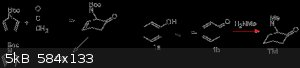
Okay, recently I figured that a double Michael addition of methylamine to 2,6-cycloheptadienone [1192-93-4]
https://en.wikipedia.org/wiki/Tropinone#From_cycloheptanone
So i figure if i shrink to six memebered ring get cyclohexa-2,5-dienone.
6-Methyl-6-aza-bicyclo[3.1.1]heptan-3-one
https://pubchem.ncbi.nlm.nih.gov/compound/23542376
So, i figure, this is the wrong isomer, i need keto-enol tautomer of phenol.
2,4-Cyclohexadienone
https://pubchem.ncbi.nlm.nih.gov/compound/141125
[24599-57-3] so-called "benzene one"
However, only one of the olefins can partake in a conjugate addition.
This is the so-called [4 + 1] Cycloaddition
Alternatively, another option is the unconjugated olefin could be replaced by a leaving group such as bromide or tosylate?
[Edited on 22-3-2021 by Illegal Parkinson]
|
|
|
Illegal Parkinson
Hazard to Self
 
Posts: 75
Registered: 2-10-2005
Member Is Offline
Mood: No Mood
|
|
Maybe if an amine cannot add to the diene, maybe a nitrene could work?
So conceptually if have phenol in the keto tautomer,
a 4 + 1 addition can work?
|
|
|
Illegal Parkinson
Hazard to Self
 
Posts: 75
Registered: 2-10-2005
Member Is Offline
Mood: No Mood
|
|
Okay, i thought to myself if phenol-ketone can be trapped as the acetal
this concenptually might work,
for example, tke cyclic acetal is called 1,4-dioxaspiro[4.5]deca-6,8-diene [23783-59-7].
|
|
|