Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Sythesis proposal for preparing 1,3-benzodioxole oxidation products using simple precursors
1,3-benzodioxoles are a well-known class of substituted benzene derivatives. Perhaps the most useful of these is piperanol, which can serve as a
building block for preparing many useful compounds.
Unfortunately, piperanol itself, as well as several other related compounds are difficult to obtain, because they are watched precursors because they
can be used in the synthesis of the entactogen MDMA, which is a controlled substance.
Unfortunately this means that much legitimate chemistry with these compounds is difficult to perform. But unfortunately I doubt that the people
drafting these rash laws care or even realize the wider consequences for science...
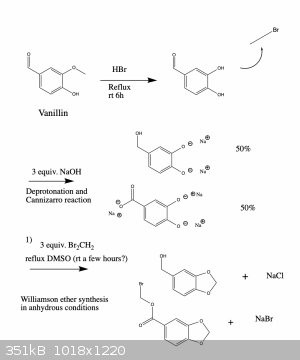
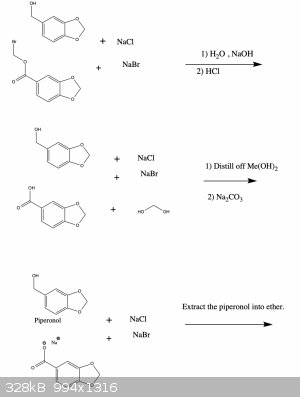
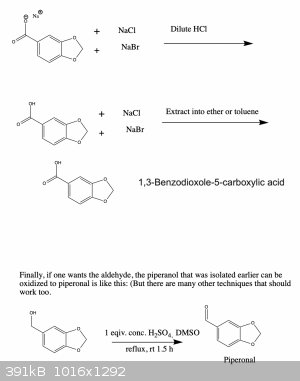
Anyway, I've attached a possible synthesis scheme to make oxidized 1,3-benzodioxoles from the readily available compound vanillin. I haven't tried
this and since I'm a bit squeamish I'm probably not going to anyway (for the reasons I mentioned above), but I'd be curious to hear your thoughts
about the feasibility of this synthesis. If you see any errors I made let me know and I'll fix it right away. Cheers 
[Edited on 12-6-2021 by Monoamine]
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
I mean, I can appreciate that you worked hard on this, but a lot of the chemistry here isn't that simple, or is just incorrect. Demethylation of
vanillin, your very first step, is often shown to be incredibly frustrating and low-yielding, and attempts made with hydrobromic acid as you suggest
frequently don't demethylate it, and those that do show signs of hope often lead to brominated products as well.
A Williamson ether synthesis doesn't involve a carboxylic acid; it involves a deprotonated alcohol, such as a phenol or an alkoxide in anhydrous
conditions. So one of your products in the second picture is incorrect. Dihalomethanes aren't going to react in any way with piperonylic acid, if one
were to get that far in the first place.
I'm not sure why you propose carrying out a Cannizzaro reaction after demethylation of vanillin, when piperonal can be made directly from
protocatechualdehyde and then someone could use piperonal itself as the substrate for cannizzaro, if they actually want piperonyl alcohol and
piperonylic acid.
|
|
|
Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Quote: Originally posted by Amos  | I mean, I can appreciate that you worked hard on this, but a lot of the chemistry here isn't that simple, or is just incorrect. Demethylation of
vanillin, your very first step, is often shown to be incredibly frustrating and low-yielding, and attempts made with hydrobromic acid as you suggest
frequently don't demethylate it, and those that do show signs of hope often lead to brominated products as well.
A Williamson ether synthesis doesn't involve a carboxylic acid; it involves a deprotonated alcohol, such as a phenol or an alkoxide in anhydrous
conditions. So one of your products in the second picture is incorrect. Dihalomethanes aren't going to react in any way with piperonylic acid, if one
were to get that far in the first place.
I'm not sure why you propose carrying out a Cannizzaro reaction after demethylation of vanillin, when piperonal can be made directly from
protocatechualdehyde and then someone could use piperonal itself as the substrate for cannizzaro, if they actually want piperonyl alcohol and
piperonylic acid.
|
Thank you for your insights and for pointing out these flaws. Is the demethylation step law yielding for vanillin specifically? The reason I'm asking
is because I've successfully cleaved aromatic methyl ethers in the past using this approach (the nice thing is that the MeBr produced is a gas and so
leaves the vessel, which should drive the reaction to completion). However, I tried this with indole as the aromatic ring (specifically with
5-methoxyserotonin) to form serotonin. So I suspect that it might work here too, unless there is something fundamentally different about this reaction
when it is performed with vanillin.
Please excuse my lack of knowledge about the Cannizzaro reaction. As far as I can understand, threading and aldehyde with OH- will cause
this reaction to occur. But I don't know if this requires a high temperature or not. If you know, I would be grateful for your advice. Also, if
there's a non RO- base that is strong enough to deprotonate phenol (possibly an alkylamine, the pKa of phenol is about 10 and the pKa of
methylamonium is about 10.63 refference), might this be an option?
Thanks also for pointing out that carboxylic acids don't form ethers via Williamson ether synthesis.
It looks overall this can be simplified somewhat: 1) If the Canizzaro reaction can be avoided, then we basically get piperonal as the sole product,
right? 2) If then we can skip several steps and get right to the carboxylic acid.
If you think this is right let me know and I'll post an updated version.
Cheers!
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The Cannizzarro reaction is not really a useful preparative method. If you want an alcohol, you use a reduction. If you want an acid, you use an
oxidation. You almost never want a 50/50 mixture of the two. It is more of a warning: do not combine aldehyde and alkali, or you will get this crap.
Also, you can absolutely esterify a carboxylate with an alkyl halide. You just can't do it with dibromomethane, because it isn't electrophilic enough.
Unless you use the silver salt, because weird silver stuff.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Go to the search engine... Search!
There have been many discussions on this subject.
Find out what is likely work. No point in trying to re-invent the wheel.
|
|
|
Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Errata
So for some reason, the system won't let me correct the original post, so consider this the errata to it.
Here is an updated synthesis proposal, which hopefully would give piperonol and two of its oxidized products.
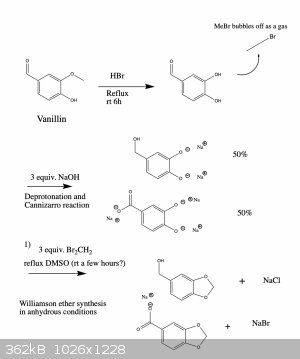 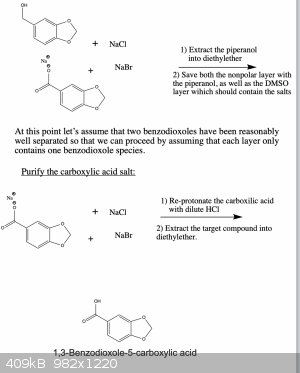 
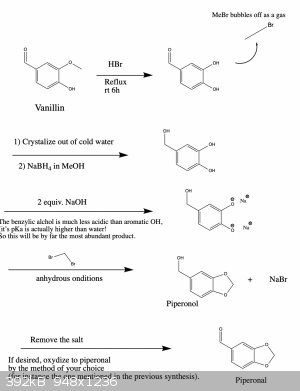
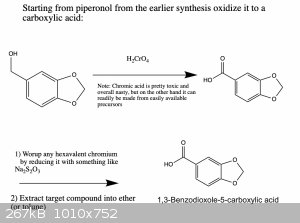
I wrote it like this so that one can get all three products if needed. You're right, if you only want one of them there are more straightforward ways
that could work. However, the ones that I know of suffer from the disadvantage that they use reagents which are harder to obtain or which are more
dangerous and toxic to work with. But in any case, below are two schemes that should work.
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
| Quote: | | The Cannizzarro reaction is not really a useful preparative method. If you want an alcohol, you use a reduction. If you want an acid, you use an
oxidation. You almost never want a 50/50 mixture of the two. It is more of a warning: do not combine aldehyde and alkali, or you will get this crap.
|
I wouldn't say it's entirely a bad idea; I've intentionally done the reaction of benzaldehyde to benzyl alcohol and benzoic acid merely as a test of
my bench chemistry skills, and it was high-yielding enough that I would do it again on other aldehydes that are cheap and easy to obtain. I'll
probably eventually do a bulk preparation of vanillyl alcohol and vanillic acid starting from vanillin someday. I'd love to see what kinds of
delicious-smelling esters could be made from them.
| Quote: | | Also, you can absolutely esterify a carboxylate with an alkyl halide. You just can't do it with dibromomethane, because it isn't electrophilic
enough. Unless you use the silver salt, because weird silver stuff. |
That reaction wouldn't be a Williamson synthesis though. And I said that dihalomethanes wouldn't react, not all alkyl halides.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | | That reaction wouldn't be a Williamson synthesis though. And I said that dihalomethanes wouldn't react, not all alkyl halides.
|
I wasn't responding to you, I was trying to clarify an apparent misunderstanding of Monoamine. If we are going to be instructors, we need to get the
right message across. But yes, it would be somebody else's esterification, I think. 
So for another boring side point: if you are going to demethylate vanillin with HBr, you should probably be using HBr in dry acetic acid, not aqueous
hydrobromic acid. The former system is much more active, and as others have mentioned, vanillin is a resistant substrate.
The 4-hydroxy on vanillin also inhibits the Cannizzarro reaction due to keto-enol tautomerism. The reaction is still possible, but takes a long time.
However, heating solutions of catecholate dianion for a long time can lead to aerobic oxidation and consequent polymerization. This inhibition of the
Cannizzarro happens to also be key to the prep of syringaldehyde and myristicinaldehyde from vanillin.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Depending on substrate, yields on the Cannizzarro, may not be 100%. In some cases there are side reactions that decrease the yields of usable
products substantially.
Aryl ethers other than methyl, are easier to cleave.
Seriously, if you are interested in this subject; utilize the Search Engine. Spend a day or so, reading-up on the subject of Heliotropin synthesis.
In my opinion, he yields that most folks have actually achieved, are disappointing, and were I hot to get myself into trouble... I would purchase a
quantity of the 3,4-Methylenedioxyacetonitrile as a starting material.
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
If you’re looking for a phenolic dealkylation procedure then take a look at this paper. The most OTC phenolic ether cleavage method that I’ve seen
uses aluminum halides to form an aluminum phenolate intermediate that gets broken during acidic work up. Another way you might go about piperonal
preparation is glyoxylic condensation with 1,3-benzodioxole. I’ll attach both papers here for you to scroll through.
Edit: issue with uploading files
Attachment: tian2018.pdf (463kB)
This file has been downloaded 298 times
[Edited on 15-6-2021 by Opylation]
Attachment: 1488-Bjorsvik.Liguori.Minisci.Synthesis.Vanillin.Isovanillin.Heliotropin160c.pdf (183kB)
This file has been downloaded 266 times
|
|
|
Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Quote: Originally posted by Opylation  | If you’re looking for a phenolic dealkylation procedure then take a look at this paper. The most OTC phenolic ether cleavage method that I’ve seen
uses aluminum halides to form an aluminum phenolate intermediate that gets broken during acidic work up. Another way you might go about piperonal
preparation is glyoxylic condensation with 1,3-benzodioxole. I’ll attach both papers here for you to scroll through.
Edit: issue with uploading files
Thank you sir!
[Edited on 15-6-2021 by Opylation] |
|
|
|