| Pages:
1
2
3 |
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
furfural
For those of you who love heterocyclic compounds I will describe my experiment in making furfural. I used the procedure in the forum library in
Gattermann (1937).
300g of oat bran was used as substrate. This was steeped in dilute sulfuric acid then steam distilled yielding 700mL of distillate. The residue in
the pot was char, easily removed. The distillate was neutralized with Na2CO3 then saturated with salt and distilled, collecting 300mL. This
distilllate was then salt saturated again and extracted with 50mL of ether. The ether was removed by simple distillation leaving 1-2mL of crude
furfural in the pot, not enough to purify. It gave a strong postive Tollen's test (silver mirror) for aldehyde. It has a subtle and pleasant smell
of coffee and almond. If I ever do this again I will use the procedure in OrgSyn, hopefully to get a better yield.
Questions and comments are welcomed.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
That's an interesting comparison with the Orgsyn procedure, Magpie.
The process depends on the dehydration of pentoses, so the the higher the pentose concentration in the raw material the better. Starch and cellulose
are hexoses, and don't give any furfural.
So the question becomes "what is the best OTC source of pentoses?"
Bran and corncobs have been used in the past, but is there something better?
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Xylose, if you can get it. Unfortunately its not a common thing here in the UK...
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
I had a friend make the brilliant suggestion to use ribose, available as a very questionable bodybuilding upplement, as feedstock. It's another
aldopentose and I don't see why it wouldn't work. Not as cheap as bran or corn cob and without the old-timey feel you may be going for, but compact
volumes and hopefully higher yield.
[Edited on 3-12-11 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
There are four aldopentoses: arabinose, lyxose, ribose, and xylose. Each has D- and L- configurations.
Are any of these favored in the formation of furfural, aside from availability?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  | 300g of oat bran was used as substrate. This was steeped in dilute sulfuric acid then steam distilled yielding 700mL of distillate. The residue in
the pot was char, easily removed. The distillate was neutralized with Na2CO3 then saturated with salt and distilled, collecting 300mL. This
distilllate was then salt saturated again and extracted with 50mL of ether. The ether was removed by simple distillation leaving 1-2mL of crude
furfural in the pot, not enough to purify. It gave a strong postive Tollen's test (silver mirror) for aldehyde. It has a subtle and pleasant smell
of coffee and almond. If I ever do this again I will use the procedure in OrgSyn, hopefully to get a better yield.
|
Interesting. What were the steeping conditions? Do you simply leave it to stand for some time?
Ribose may (or may not) yield higher yields of furfural but look at these internet prices! Steep (no pun intended)!
[Edited on 15-3-2011 by blogfast25]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Actually, I think "steeping" is not really required here and is my misnomer; it was not called for in the procedure. Steam distillation conditions
that are used to separate out the mash are probably all that is required to hydrolyze the pentosans. I let the mix of dilute sulfuric and bran sit
overnight merely for my convenience.
I've done some pricing of d-ribose and the best I've seen is about $11/100g. So if you wanted a quantity of furfural that seems like the way to go.
I was surprised how reactive it was in the Tollen's test - put benzaldehyde to shame. No heat was required for either, however.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
jimwig
Hazard to Others
  
Posts: 215
Registered: 17-5-2003
Location: the sunny south
Member Is Offline
Mood: No Mood
|
|
CORNCOBS r cheap
CORNCOBS r cheap
craZy jiM wGGns
--packrat, professional bum. -- once just tired
now REtired.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Giant bloody flasks and heat sources to hold the volume needed to make comparatively small amounts of furfural with them are not.
[Edited on 3-16-11 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thanks Magpie…
Far more interesting than furfural would be the famous derivatives of THF. I suppose the hydrogenation is outside the capability envelope of most of
us here?
The price of Ribose must be somewhat artificially inflated by its primary market. These guys and galls will pay virtually anything!
And how about d-fructose: considerably cheaper than ribose. Wiki:
"DehydrationFructose readily dehydrates to give hydroxymethylfurfural ("HMF"). This process may in the future be part of a low-cost,
carbon-neutral system to produce replacements for petrol and diesel from plantations."
Ah... unleaded HMF, super! 
[Edited on 16-3-2011 by blogfast25]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
It is interesting that the Gattermann procedure says to expect a yield of 6-7g on 300g of oat bran. This is 0.020-0.023g/g. The OrgSyn procedure,
however, says to expect 165-200g/1.5kg of corncobs. This is 0.110-0.133g/g. This a 5-fold yield for the corncobs over the oat bran.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Ribose will form furfural on heating with acid;
http://www.cerlabs.com/experiments/10875404464.pdf
If you wanted a few grams of fufural I think this would be the way to go, 25g of ribose reacting in a 50ml flask to yield maybe 10g grams of furfural
sounds way more tempting.
|
|
|
gsd
National Hazard
   
Posts: 847
Registered: 18-8-2005
Member Is Offline
Mood: No Mood
|
|
Furfural from Corncobs
A set of 4 articles on this subject appeared in I & EC in 1923-24
by Frederick B. LaForge and Gerald H. Main
I—Factors Influencing the Furfural Yield in the Steam-Digestion Process
II—The Bureau of Chemistry Experimental Plant and Process for Furfural Production
III—Effect of Catalysts on Furfural Yield in the Steam Digestion Process
IV—Economic Aspect of Furfural Production
They are attached below.
Besides this I remember there was an excellent article on comparative study of furfural yields from several pentosan sources such as Oat meal, corn
cobs, groundnut husk, rice husk, sugarcane baggase etc. in one old issue of J Chem & Met Engg, which unfortunately is not on the net. I have a
photocopy somewhere but it will need lot of digging.
gsd
Attachment: Furfural from Corncobs-I.pdf (158kB)
This file has been downloaded 1141 times
Attachment: Furfural from Corncobs-II.pdf (581kB)
This file has been downloaded 3550 times
Attachment: Furfural from Corncobs-III.pdf (154kB)
This file has been downloaded 1330 times
Attachment: Furfural from Corncobs-IV.pdf (159kB)
This file has been downloaded 2835 times
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Quote: Originally posted by blogfast25  | Thanks Magpie…
Far more interesting than furfural would be the famous derivatives of THF. I suppose the hydrogenation is outside the capability envelope of most of
us here?
|
You never know. Furfural is not a highly aromatic system to the point that strong acid will cleave the ring. It should be much more readily
hydrogenated than say, benzene. You probably will not get the aldehyde to a methyl using any easy lab conditions.
IIRC rhodium catalysts can reduce even benzene rings at room temp and 1atm (I may be wrong, don't quote me on it) so they would be great for this.
[Edited on 3-16-11 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
For those really into sugar chemistry I see that d-arabinose can be made from calcium gluconate. There is a procedure for this in the forum library
that I can't find at the moment. Calcium gluconate is about 1/4 the cost of d-ribose. IIRC arabinose is an aldopentose and should, like ribose,
yield furfural upon hydrolysis.
Edit:
Here it is:
http://library.sciencemadness.org/library/books/an_advanced_...
In fact, you can even start with glucose, as the procedures show.
[Edited on 30-3-2011 by Magpie]
[Edited on 30-3-2011 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
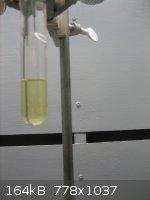
Disappointed with the yields of my previous experiments in making furfural, I tried again, this time with fairly good success.
I used a 1/10th scale version of the Org Syn procedure, substituting d-ribose for corn cobs. My pot was a 1000mL RBF with a Hempel column and
whatever else I could find to build a long vertical tube. The receiver is a 25mL side-arm test tube. A picture of the assembly is shown below. It
uses a Dean Stark mechanism to separate the furfural from the aqueous phase. Density of furfural is 1.159 @13C so it separates nicely from the water.
Solubility in water is 9.1g/100, so it is advantageous to use as small a receiver vessel as possible.
I charged the pot with 500mL of 2.17N H2SO4, 200g NaCl, and 32g of food grade d-ribose. This was then set to boiling for 3 hours. As usual the pot
contents turned black once boiling temperature was reached. So, all that carbon has to be considered a loss in yield. 8mL, or ~9.3g, of crude
furfural was recovered as a translucent yellow liquid. This is a yield of 45.3% based on the d-ribose charged.
Questions, comments, and discussion are welcomed.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
I think you might do a little better if you added some salt to the collection vessel. The solubility of organic chemicals in water is affected by the
presence of other disssolved materials.
Also extraction with ether would probably get some more of the furfural out of product.
Was there substantial amounts of black material or was it just colouration?
A little black goo goes a long way! 
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by ScienceSquirrel  | I think you might do a little better if you added some salt to the collection vessel. The solubility of organic chemicals in water is affected by the
presence of other disssolved materials.
Also extraction with ether would probably get some more of the furfural out of product.
Was there substantial amounts of black material or was it just colouration?
A little black goo goes a long way!  |
The small amount of water in the reciver (<20mL) becomes saturated with furfural early in the distillation. From then on the furfural just drops
out and settles on the bottom of the test tube. The water in the tube is continually being dribbled back to the pot and refreshed with incoming
distillate. I agree that at the end of the distillation I should be able to drive the 8% or so of dissolved furfural out of the water by adding salt.
Yes, ether would be a good solvent but then there is the mess and stink of handling it. On this small scale I don't see it being worth the effort.
As you say, a little black goo goes a long way. I just emptied this flask. Because of the remaining salt it is a little hard to quanitfy the amount
of carbon. Several grams is about all I can say.
In August when Mrs Magpie cooks "sweet corn," ie, "corn-on-the-cob," I may give corncobs a try.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
Excuse me, but what is the fuss about furfural?
Are you fellas into biomass and biofuels, or what am I missing here?
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
I think you are right and there is little more to be gained.
It is a complex condensation reaction and the chance of any one molecule of ribose twisting around to the right conformation, eliminating and
condensing in the right reaction order to make furfural or making goo may have a statistical limit.
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
It's feedstock into furan, and from there to tetrahydrofuran
(THF) and others.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by ScienceSquirrel  | ...
It is a complex condensation reaction and the chance of any one molecule of ribose twisting around to the right conformation, eliminating and
condensing in the right reaction order to make furfural or making goo may have a statistical limit. |
That is an interesting way to look at it. Indeed, each ribose molecule must lose 3 molecules of water to form furfural. I too was wondering why so
much of the ribose ends up as carbon. At first I was using a much stronger acid, even sulfuric, which is known for its tendency to carbonize. But
this last batch was only 2.17N in H2SO4, or HCl, however you want to look at it. OrgSyn, however claims a very high yield when using corncobs. We'll
see how I do with those in August.
@GreenD: I'm making furfural just for the heck of it. It's an interesting looking aldehyde, and being able to make it from corncobs, straw, oat
hulls, etc, just adds to the intrigue.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Arthur Dent
National Hazard
   
Posts: 553
Registered: 22-10-2010
Member Is Offline
Mood: entropic
|
|
@ Magpie: Quick question, what do you do to the corn cobs to prepare them into a primordial soup? Just dilute sulfuric acid? How long does it take to
boil off? Corn cobs are pretty sturdy things, so I figure it might be a long process to digest those...
- Robert
--- Art is making something out of nothing and selling it. - Frank Zappa ---
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Robert, here's the procedure as found in OrgSyn. For those who don't know, this cornocopia (no pun intended) is freely available to the public. 
http://www.orgsyn.org/orgsyn/default.asp?formgroup=basenpe_f...
Yes, the cobs apparently can take 5-10 hrs to digest.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
A very interesting experiment, Magpie, look forward to the details on the corn-on-the-cob run.
If only there was an easy way to convert to THF. Or is there?
How much did the ribose set you back?
|
|
|
| Pages:
1
2
3 |