Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Replacing halogen by hydrogen
I've been looking far and wide for a method to perform the following reaction:
R-O-CH2-X -> R-O-CH3
In other words, all I'm trying to do is get rid of a Cl- and replace it with an H-.
Classical reducing agents such as NaBH4 or Li AlH4 were never mentioned as a means to achieve this reaction.
On the other hand, would't it just be as simple as using LiH or NaH so that the Na+ undergoes nucleophilic attack by Cl- and, while the resulting
carbon cation undergoes nucleophilic attack by H-, yielding:
R-O-CH2-X -> (NaH) -> R-O-CH3 + NaCl
Would this work, or is there maybe a much more streight forward way to achieve this?
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
I’m not sure what the scope is (I’ve only used it on chlorines that are alpha to a ketone) but at work I’ve de-chlorinated compounds using
chromium(II) chloride. Downsides are the chromium(II) chloride is not too easy to come by or make, and you have to use a stoichiometric amount.
Really, a considerable excess if you want to ensure it goes to completion.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Pick one.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Monoamine  | I've been looking far and wide for a method to perform the following reaction:
R-O-CH2-X -> R-O-CH3
In other words, all I'm trying to do is get rid of a Cl- and replace it with an H-.
Classical reducing agents such as NaBH4 or Li AlH4 were never mentioned as a means to achieve this reaction.
On the other hand, would't it just be as simple as using LiH or NaH so that the Na+ undergoes nucleophilic attack by Cl- and, while the resulting
carbon cation undergoes nucleophilic attack by H-, yielding:
R-O-CH2-X -> (NaH) -> R-O-CH3 + NaCl
Would this work, or is there maybe a much more streight forward way to achieve this? |
If there's nothing else that would react (that's a big if) I think you can go via a Grignard reagent or some such.
|
|
|
Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Ah cool, Grignard may be the way to go then. Thanks for the tip!
Basically, I was hoping to make a methyl ether, but the problem is that methyl halides tend to be gasses, so I don't know if I would have enough...
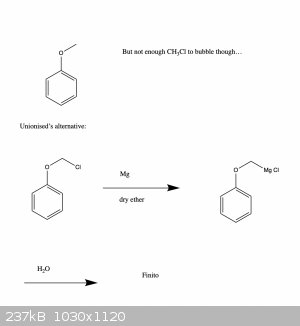
I guess I could try to reflux some MeOH and HBr and bubble the fumes through the deprotonated phenol, but not really sure how efficient that would
be..., hence the roundabout route.
I've been meaning to get some practice with Grignard reagents anyway, so this also seems a good way to practice.
[Edited on 31-7-2021 by Monoamine]
|
|
|
Oxy
Hazard to Others
  
Posts: 140
Registered: 1-12-2020
Member Is Offline
|
|
Methyl iodide has a boiling point of 42oC
Here you can find some info about alpha-haloalkyl ether's chemical properties
Attachment: summers1955.pdf (3.2MB)
This file has been downloaded 192 times
[Edited on 31-7-2021 by Oxy]
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
SmI2 in THF or THF/HMPA can reduce alkyl halides to hydrocarbons.
https://sci-hub.se/https://pubs.acs.org/doi/10.1021/ja00528a...
https://books.google.cz/books?id=QUfQamGsEgwC&printsec=f...
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
Fine: an excess. Either way, my point was that you have to use at least a stoichiometric
amount of something that is not so easy to come by, and if you want it to work really well, you'll need to use an excess. So although it is a cool
reaction, it is likely not viable in the home lab.
If it was possible to use a catalytic amount of CrCl2 with an excess of a secondary reductant like zinc metal, that would be a different
story, but I don't think that works.
[Edited on 7-31-2021 by Texium]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
You could react that stuff with borohydride and CuCl2, that would dehalogenate it.
I don't know about other function groups in your molecule though.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Remember that a stoichiometric amount of CrCl2 for this rxn is two molar equivalents!
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Monoamine: If you want to make anisole from phenol, you can use dimethyl oxalate as methylating agent:
http://www.sciencemadness.org/talk/viewthread.php?tid=86622
|
|
|