sherlocked
Harmless

Posts: 2
Registered: 9-4-2019
Location: UK
Member Is Offline
|
|
Retrosynthesis
Hi guys.
I am an undergrad student studying organic chemistry and I have a retrosynthesis problem I am having trouble with. I am hoping you guys could help me
out. The instructions are to return to starting materials that have 6 carbons or less that are either mono or di functionalized.
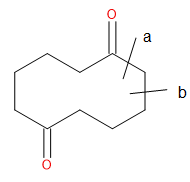
As you can see in the image posted I can either break the c-c bond at 'a' or 'b'. either way I will form a 1,6 dicarbonyl chain. I am not sure which
would be ideal. after this I have decided on a reconnection to a substituted cyclohexene which could be broken via ozonolysis. From this I can FGI to
the substituted cyclohexanol which can further be disconnected to give the cyclohexanone and a grignard reagent.
Would do you guys think? My professor said that it could be synthesized a better way. Thanks in advance!
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
How about adipic acid plus a grignard?
Or would that just polymerize?
|
|
|
sherlocked
Harmless

Posts: 2
Registered: 9-4-2019
Location: UK
Member Is Offline
|
|
A Grignard on a carboxylic acid? Would that not just protonate the Grignard reagent?
If I were to use the Grignard on the acid chloride or ester of adipic acid I would also get 2 additions of the Grignard reagent which isn't what I
need.
perhaps I am misunderstanding your suggestion. Thanks for the help.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
No, I was just wrong.
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Your target is cyclodecane-1,6-dione. It is typically made by ozonolysis of 1,2,3,4,5,6,7,8-octahydronaphthalene. Knowing this as a last step, your
problem reduces to the synthesis of a rather well known bicyclic target. A bit of literature searching will lead you to methods of synthesis for the
1,2,3,4,5,6,7,8-octahydronaphthalene precursor. Have fun - you will enjoy the learning.
AvB
|
|
|