Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
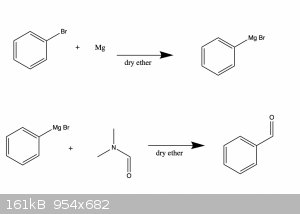
Question about Grignard reaction
I'm going to try out a Grignard reaction for the first time soon and I want to make sure I get it right since I've never tried it before. I'm going to
try to make an aldehyde from an aryl-bromide.
In principle this can be done by reacting the aryl-bromide with magnesium in dry ether at room temperature. This is then followed by reacting the
Grignard reagent with dimethylformamide (DMF) in dry ether at -5C-0C.
I was wondering a few things:
1) Is it enough to dry the ether and the DMF with 3A molecular sieves?
2) I only have regular freezer ice, so I think I'll only be able to cool the reaction to about 5C. Is this still cold enough to form the aldehyde with
DMF?
3) Do the amounts have to be exactly stoichiometric or is it better to use an excess of magnesium and later an excess of DMF, to ensure that the
aryl-bromide and/or the Grignard reagent are completely converted?
Thank you in advance for any answers. Also see attached reaction scheme for a simplified overview.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
1) yes, if left atleast overnight the mol seives actually give very dry solvents. Williams2010.
2) I have not done this particular reaction, but with weinreb amides 0-5 Celsius is ok. Perhaps a lower yield is obtained at 0 to 5 Celsius compared
to -20 or even lower.
3) it is better to have excess magnesium, the unreacted magnesium just sits there. It is likely better to have a small excess of DMF, although the
grignard will not be formed in quantitative yield so equimolar will already be a slight excess. also it might be better to add the grignard to the DMF
than the DMF to the grignard. Although both procedures are reported in the literature with comparable yield.
Interestingly this ancient paper describes the reaction of phenyl grignard and DMF to mainly produce side products.
Attachment: smith1941.pdf (368kB)
This file has been downloaded 206 times
Attachment: williams2010.pdf (772kB)
This file has been downloaded 166 times
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
You can put a solution of CaCl2 in your freezer, this will stay liquid but will be as cold as your freezer gets. Maybe first precool with regular ice,
then put in the CaCl2 solution.
Magnesium/bromobenzene will react at room temperature, but the reaction is exothermic. Don't add all bromobenzene at once, add a little until the
reaction starts, which might even require some heat. Use an efficient reflux condensor, especially when working with ether.
It helps when you threat the magnesium with some 1% HCl, half a minute or so. The rinse with something cheap, like methanol, then with a little ether.
A crystal of iodine also works to get the reaction started.
[Edited on 29-12-2021 by Tsjerk]
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 195
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
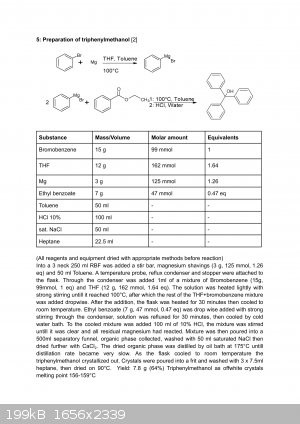
Aryl halide grignard reagents are particularly suited for Toluene+THF solvent system, which in my opinion is superior to diethyl ether, since the
boiling point is substancially higher, making the reagent formation easier to activate aswell as making dealing with the exotherm easier. The reagent
in this solution is also proven to be kinetically more reactive compared to just ether as the solvent. Amazingly, even chlorobenzene forms the reagent
in toluene solution. (further info look up "grignard reagents in toluene solutions : https://doi.org/10.1002/aoc.333")
Here is a preparation i performed using that solvent system, dont mind the low yield as this was only my second time ever performing a grignard
reaction, yields with quality reagents and good practice are the same as with just ether:
As for your questions:
1) yes
2) As Tsjerk said, calcium chloride an crushed ice can reach temperatures as low as -40C (~ mass ratio 2.4:1 CaCl2:ice for that)
3) excess of Mg and and substrate is appropriate, ideally by addition of the grignard reagent to the DMF, but thats not so easy to do without letting
the reagent contact air, so you might just have to add the DMF to the grignard reagent unless you have needles and septa to perform the transfer air
free.
[Edited on 29-12-2021 by RustyShackleford]
|
|
|
Dr.Bob
International Hazard
    
Posts: 2656
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
The first step is a classic Grignard starting material. The bromobenzene is one of the best halides for doing a Grignard. I would dry the solvent
as mentioned above, then put the Mg turnings (2 eq is typical) in the flask, maybe grind it in a mortar and pestle first or just bang them with a
hammer or file them some. Anything to make clean surface area. Washing with dilute acid works, but them you should wash with water, and then
acetone or ether and dry the Mg under vacuum well, as any water will be a bad thing.
Add the ether to the Mg, then add 10-20 % of the bromide and stir a while, vigerously. After a while, you can start adding the remaining bromide
dropwise if possible. If the reaction starts boiling, then slow down the addition to control the boiling/reflux. The slower you add and the
longer you let it stir, the better (I often let them stir overnight, or even reflux them a while once they appear done), but if you don't have a
source of inert gas, and drying tubes, then water will slowly seep into the reaction and quench it.
If you only have ice, you can add some salt, CaCl2, or other salts to cool the reaction below 0 C. It depends a lot on the scale, the bigger it is,
the more helpful good cooling is. But the larger the scale, the less effect of trace water, CO2, and other contaminanents. And a slight excess of
DMF is ideal. You can later distill the aldehyde from it, or just leave it there for many reactions. When done, it is typical to pour the
reaction into ice water and wash with acid to quench any remaining Grignard and break down the Mg salts formed. Stir a 1/2 hour and then extract the
mixture with ether a few times to get the product out.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
As a partially-related question....does glyme worth as the ether for a Grignard? I know that diethyl ether and THF work quite well, but t-butyl
methyl ether apparently doesn't.
Never mind- just checked the obvious Wikipedia article, and it says it's a common solvent for Grignards.
[Edited on 29-12-2021 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Monoamine
Hazard to Others
  
Posts: 160
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Thank you for all of these valuable tips! Using a Toluene and THF sounds very useful. Also thank you for the tips on cooling. Also thanks for the tips
on which order to add the reagents to each other (I probably would have messed that part up...)
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Grignard reactions are really not that hard, don't worry.
Molsieves and a tiny bit of iodine are already guarantees that it will work.
Even without all the fancy stuff like a drying tube, a pressure equalized addition funnel etc... nah, just a piece of aluminium foil on top of the
condenser suffices if you use plain ether or THF 
@DraconicAcid: MTBE does work as a solvent for the addition, but not as a solvent for the formation of the reagent.
But you can safely add whatever component in MTBE as soon as your reagent is formed.
Similar to toluene or the like.
The selectivity is only noteworthy and important for forming the reagent, but using them is possible in a much broader selection of solvents.
All in all, grignard reactions, or how complicated they are, are greatly overestimated.
Bwiti at the Hive ran one into a mason jar in his kitchen, successful.
So don't fret, its just a grignard reaction.
Not rocket science 
verrückt und wissenschaftlich
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by karlos³  |
@DraconicAcid: MTBE does work as a solvent for the addition, but not as a solvent for the formation of the reagent.
But you can safely add whatever component in MTBE as soon as your reagent is formed.
Similar to toluene or the like. |
True, but it's the formation of the RMgX that's important.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|