Z4x
Harmless

Posts: 8
Registered: 16-10-2010
Member Is Offline
Mood: No Mood
|
|
NaCl Crystals
Hi,
I would like to make some nice large NaCl crystals. I played with this in the past and started again the last few days.
Below are some pictures of the latest NaCl crystals, but growing too fast. Still pretty I think. Old NaCl crystals I have grown: https://commons.wikimedia.org/wiki/User:Choba_Poncho
Can you help me with information? I'm thinking about using concentration difference by temperature instead of evaporating water (what I use now).
Topics, papers, website, industrial method?, please post them.
Thanks.
If a larger crystal will not work, I might go back to small ones with a macro lens. Is a bit easier. 
 

|
|
|
Rainwater
National Hazard
   
Posts: 798
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
https://youtu.be/FKCS1DvORug
Crystallization is. The slower, the bigger.
You can control the temperature with a heater and insulation.
Evaporation by limiting free air movement.
The basic principle is
when a solution is super saturated, they compounds will begin to percipitate out if they have a neculation site.
Here are some overkill examples. If your gonna do it, do it to 1000th of an inch
Example 1,
if we have 38.99g of the compound NaCl.
A very clean container.
We add 100mL of pure water.
Assuming amount of water in the container will not change throughout the experiment we will heat the solution to 100c, and dissolve all of the salt.
Then, a seed crystal suspended in the container, to act as a neculation site, then the container is sealed.
https://en.m.wikipedia.org/wiki/Solubility_table
0c - 35.65
100c - 38.99
In example 1, if we very slowly cool the solution down to 0c. Over the course, let say 24 hours. We can expect to percipitate 3.44g of salt onto our
seed.
I don't know how long this process should take. I'm guessing at the time frame.
If you have a dirty container or cool too quickly, other crystals will begin to form and steal from your yield.
Example 2.
Same conditions, but instead of changing the heat, we will change the volume of the solution.
Use a heater to maintain the temperature of the solution.
Set up an air pump into the sealed container inlet with a dryer and let the outlet go to the atmosphere. Both the inlet and outlet have check valves
to create an ideal environment inside of the container
At regular intervals, run the air pump for a short time.
In example 2, we use a controlled method of evaporation. As the air sits inside the container it absorbs water. When the pump runs, the wet air is
replaced with dry air. Allowing more evaporation. As the volume of the solution decreases, salt will precipitate out, because of the constant
temperature, the amount of salt per 100ml stays the same. So as the volume of solution decreases more salt will fall out.
Its best to use pure chemicals when growing crystals. Rapid recrystallization will produce small and pure crystals. Use them to create the solution
for your large crystals.
"You can't do that" - challenge accepted
|
|
|
Twospoons
International Hazard
    
Posts: 1280
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
This might interest you. Its an experiment I did growing CuSO4 crystals using a thermal differential method. It produced quite a large crystal
in a couple of weeks.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 163
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Oh man I love those.
https://cdn.discordapp.com/attachments/930901959605616706/94...
If you add some glycerol to the solution it seems to improve the transparency of the resulting crystals.
[Edited on 3/2/2022 by SnailsAttack]
|
|
|
Z4x
Harmless

Posts: 8
Registered: 16-10-2010
Member Is Offline
Mood: No Mood
|
|
Thank you all for the replies.
Quote: Originally posted by Twospoons  | | This might interest you. Its an experiment I did growing CuSO4 crystals using a thermal differential method. It produced quite a large crystal
in a couple of weeks. |
Wow, very interesting technique and nice results. Elegant closed system. Do you know more about this? Do dimensions matter? I presume this works very
nice for CuSO4 because it dissolves a lot by higher temperature. NaCl dissolves a lot less, will it still work?
Nice crystals. The ones on the wall almost look like something else... iron based salt?
I did not know glycerol improves the transparency of NaCl crystals. I'm going to try that right away. Is there a reason why? Where did you learn this?
Do you have more information? How much too add?
[Edited on 4-3-2022 by Z4x]
|
|
|
Morgan
International Hazard
    
Posts: 1660
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I liked these cubes.
Dead sea amazing natural phenomenon - DeadSea salt cubes
https://youtu.be/Nw0aY3SXIdg
|
|
|
Twospoons
International Hazard
    
Posts: 1280
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
The closed hydrothermal system only requires different solubility at different temperatures - which NaCl has. I don't believe dimensions are that
critical, though there are obvious extremes that you would expect to perform poorly. So long as you can create a thermal gradient between your source
material and your crystal I think it should work. I didn't do any calcs for mine, I just used what I had on hand and hoped for the best. I'm certain
its far from an optimal setup, but it clearly does the job. There is a lot of room for optimizing the setup - the simplest being a variable heat
input to control the growth rate. Something you might want to consider for NaCl, since it seems better crystals result from slower growth. One big
improvement would be ensuring a stable environment - you don't want the whole thing heating and cooling on a daily basis.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
Z4x
Harmless

Posts: 8
Registered: 16-10-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Twospoons  | | The closed hydrothermal system only requires different solubility at different temperatures - which NaCl has. I don't believe dimensions are that
critical, though there are obvious extremes that you would expect to perform poorly. So long as you can create a thermal gradient between your source
material and your crystal I think it should work. I didn't do any calcs for mine, I just used what I had on hand and hoped for the best. I'm certain
its far from an optimal setup, but it clearly does the job. There is a lot of room for optimizing the setup - the simplest being a variable heat
input to control the growth rate. Something you might want to consider for NaCl, since it seems better crystals result from slower growth. One big
improvement would be ensuring a stable environment - you don't want the whole thing heating and cooling on a daily basis. |
I tried the following:
Closed 1.5L water bottle with concentrated NaCl solution.
Bottom NaCl crystals, slightly above it heating wire. Around 4 watt.
Aluminium blanket at the bottom to keep heat in.
At top a cooling fan and a crystal hanging in the solution by a nylon wire.
The crystals at the top keep dissolving. Even after letting the system stabilize for a few days. 
It's like the system is working the wrong way, haha.
The water had some dust in it and so I could see it moving with a bright light.
Schematic image:
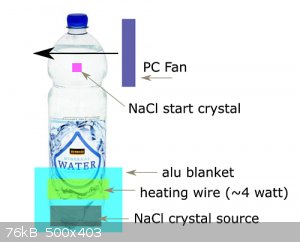
The water bottle is rather wide compared to its height. So cooling surface vs volume not optimal. Could I perhaps simulate the heat/mass transfer
somehow?
.
[Edited on 13-3-2022 by Z4x]
|
|
|
Rainwater
National Hazard
   
Posts: 798
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Sounds like the solution isnt saturated
"You can't do that" - challenge accepted
|
|
|
Twospoons
International Hazard
    
Posts: 1280
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
Agreed. I start with a hot saturated solution in the setup, then let that thermally stabilize with the heater on over a day or two. A lot of salt will
crystalize out on the bottom as the solution cools to equilibrium. Only then do I put the seed in at the top.
The fan at the top probably isnt needed - you don't need a huge thermal gradient.
How stable (thermally) is the environment? If the whole thing warms up on a hot day, that could also dissolve your seed.
You need to filter that dust out too - if any settles on your seed it could create additional crystals, and you wont get a nice cube.
I would also put the heater under the bulk NaCl, rather than above it.
[Edited on 13-3-2022 by Twospoons]
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 163
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by Z4x  |
Nice crystals. The ones on the wall almost look like something else... iron based salt?
I did not know glycerol improves the transparency of NaCl crystals. I'm going to try that right away. Is there a reason why? Where did you learn this?
Do you have more information? How much too add?
|
The offcolors are caused by dye in the glycerol. It's low-purity mouthwash. I don't know why it improves the transparency.
https://en.crystalls.info/Sodium_chloride
This is where I read about it. Technically it says "glycine" but that's most likely a typo.
[Edited on 3/15/2022 by SnailsAttack]
|
|
|
Twospoons
International Hazard
    
Posts: 1280
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
I want to retract my comment about not needing the fan - NaCl has such a flat solubility vs temp curve you probably do need to create a sizeable
thermal gradient for this to work.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Quote: Originally posted by Z4x  | Hi,
I would like to make some nice large NaCl crystals. I played with this in the past and started again the last few days.
Below are some pictures of the latest NaCl crystals, but growing too fast. Still pretty I think. Old NaCl crystals I have grown: https://commons.wikimedia.org/wiki/User:Choba_Poncho
Can you help me with information? I'm thinking about using concentration difference by temperature instead of evaporating water (what I use now).
Topics, papers, website, industrial method?, please post them.
Thanks.
If a larger crystal will not work, I might go back to small ones with a macro lens. Is a bit easier. 
|
Hi Z4x, yes, I clearly remember the pictures you posted in the past. Great work!
Incidentally, I tried to make NaCl crystals myself 3 weeks ago. I used table salt from the supermarket without NaI, but it did contain anti caking
agent (a kind of sodium aluminium silicate). I dissolved the salt in a liter of tap water and let the anti caking agent settle over night. Then
filtered it and obtained a perfectly clear solution.
Unfortunately, when I left the solution to evaporate, on the surface of the liquid, small white crystals formed. They grew together forming a layer
which sank to the bottom of the vessel when they got too heavy. This process repeated itself all the time. No clear crystals were obtained and they
all looked white and not transparent. In addition thick crusts formed on the sides of the vessel above the liquid.
My question is: what kind of salt did you use? Was is from a chemical supplier or was it kitchen salt, like mine.
Did you use distilled water or tap water, like I did?
|
|
|
phlogiston
International Hazard
    
Posts: 1375
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
I think the problem you may be having is that the heating element heats up some solution and it starts to rise before it actually is able to become
(re)saturated at the higher temperature (because the heating element is -above- the raw salt source).
Then, this hot undersaturated solution rises by convection in the center of the bottle, passing by the seed crystal and dissolving it. The solution
then cools down at the walls and drops back down to the bottom.
Possibly, you might be able to solve it by putting the heating element under the bottle, or embedded in the salt. Also, putting the bottle on its side
might work better because it avoids that the crystal is suspended immediately above the heating element.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Probably it is not a typo. Glycine is a common compound, it is an amino acid. It is not particularly
expensive, nor very cheap, and easy to obtain. You only need a little amount.
https://www.laboratoriumdiscounter.nl/nl/glycine-99-for-synt...
http://labstuff.nl/contents/nl/d117_03.html#p3785
A very cheap source is this: https://www.ebay.nl/itm/281696164964?hash=item4196665c64:g:u...
[Edited on 17-3-22 by woelen]
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
When you have glycine you can try to grow triglycine sulfate. The crystals are nice and show birefringence.
|
|
|
Z4x
Harmless

Posts: 8
Registered: 16-10-2010
Member Is Offline
Mood: No Mood
|
|
Thank you all for the nice replies.
Quote: Originally posted by Bezaleel  |
Hi Z4x, yes, I clearly remember the pictures you posted in the past. Great work!
Incidentally, I tried to make NaCl crystals myself 3 weeks ago. I used table salt from the supermarket without NaI, but it did contain anti caking
agent (a kind of sodium aluminium silicate). I dissolved the salt in a liter of tap water and let the anti caking agent settle over night. Then
filtered it and obtained a perfectly clear solution.
Unfortunately, when I left the solution to evaporate, on the surface of the liquid, small white crystals formed. They grew together forming a layer
which sank to the bottom of the vessel when they got too heavy. This process repeated itself all the time. No clear crystals were obtained and they
all looked white and not transparent. In addition thick crusts formed on the sides of the vessel above the liquid.
My question is: what kind of salt did you use? Was is from a chemical supplier or was it kitchen salt, like mine.
Did you use distilled water or tap water, like I did? |
Thanks. It's been a while, but I think I just used plain tap water those days.
Standard NaCl food quality from the supermarket. Yeah, if the solution evaporates too fast you get crystals on the solution's surface or creeping up
on the walls.
One way to prevent that is to use new unused plastic (throw away) drinking cups. Those plastic cups show hydrophobic properties that prevent crystal
growth on the walls.
For slower evaporation you can also put the saturated solution inside a closed plastic (zip) bag with hygroscopic material in a different cup.
Now I'm looking for a different method to get faster and larger crystals... which seems to be rather difficult with NaCl (compared to CuSO4 for
example).
Quote: Originally posted by phlogiston  | I think the problem you may be having is that the heating element heats up some solution and it starts to rise before it actually is able to become
(re)saturated at the higher temperature (because the heating element is -above- the raw salt source).
Then, this hot undersaturated solution rises by convection in the center of the bottle, passing by the seed crystal and dissolving it. The solution
then cools down at the walls and drops back down to the bottom.
Possibly, you might be able to solve it by putting the heating element under the bottle, or embedded in the salt. Also, putting the bottle on its side
might work better because it avoids that the crystal is suspended immediately above the heating element. |
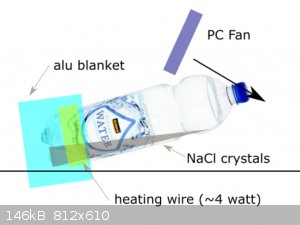
Yes, you might be right about the heating location. I like your idea of having the bottle on its side.
So I'm trying that now. See attached image. It's not completely horizontal. Perhaps there are too many NaCl crystals on the bottom, but I'm hoping to
see an area where more crystallizes.
Interesting, I might buy some to try. Internet search with NaCl and glycine showed this paper: https://www.sciencedirect.com/science/article/pii/0022024883...
I don't completely understand it, but the pH does have influence on the crystal shape: cube or rhombic dodecahedron.
Does the Glycine also get incorporated into the crystal? How bad is this? I would like at least 99.99% pure crystal. 
Quote: Originally posted by SnailsAttack  | Quote: Originally posted by Z4x  |
Nice crystals. The ones on the wall almost look like something else... iron based salt?
I did not know glycerol improves the transparency of NaCl crystals. I'm going to try that right away. Is there a reason why? Where did you learn this?
Do you have more information? How much too add?
|
The offcolors are caused by dye in the glycerol. It's low-purity mouthwash. I don't know why it improves the transparency.
https://en.crystalls.info/Sodium_chloride
This is where I read about it. Technically it says "glycine" but that's most likely a typo.
[Edited on 3/15/2022 by SnailsAttack] |
Interesting, that page also mentions that "... Growing crystal from a mixture of water and ethanol leads to forming pyramids ...". Oh, I might also
try this one day.
No mention of the ratio, so I'm a bit sceptical.
I'm also going to think about just using two containers/beakers with two different temperatures (= different concentrations) and using a simple slow
pump.
|
|
|
Z4x
Harmless

Posts: 8
Registered: 16-10-2010
Member Is Offline
Mood: No Mood
|
|
I ordered the one from ebay, but it got eventually cancelled...
| Quote: |
We hereby let you know that your recent purchase from [peak_supps] through the Worldwide Shipping Program cannot be completed.
The object is considered restricted. This may be due to import/shipping restrictions or the eligibility requirements for the Worldwide Shipping
program. The item will not be shipped to you, nor will it be returned to the seller.
Don't worry! Your full refund will be processed to the original payment method within 72 hours. You can see two separate refunds: one for the item and
one for shipping and import costs. No further action is needed. |
You guys are certain it will improve crystal quality? It feels a bit like a gamble.
Anyway... I ordered this one: https://www.bulk.com/nl/glycine.html
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Quote: Originally posted by Z4x  | Thanks. It's been a while, but I think I just used plain tap water those days.
Standard NaCl food quality from the supermarket. Yeah, if the solution evaporates too fast you get crystals on the solution's surface or creeping up
on the walls. |
I did notice them as pretty thick crusts on the walls. Layers of crystals on the surface showed all the time as well. I wasn't aware that this
behaviour is promoted by fast evaporation. It definitely makes sense, since the humidity in the room was low and the container was standing on a
windowsill over a radiator.
After all water had evaporated, I redissolved the poorly crystallised white salt and some very small amount of the anti caking agent showed up in the
liquid.
I have seen this behaviour before: when a solution contains very fine substances in suspension (so fine that it will pass through even micro pore
filters), then evaporating the solution will make these very fine particles coalesce. This also works when a lot of salt is in solution. It worked
with both BaCl2 and NaCl solutions.
Quote: Originally posted by Z4x  | One way to prevent that is to use new unused plastic (throw away) drinking cups. Those plastic cups show hydrophobic properties that prevent crystal
growth on the walls.
For slower evaporation you can also put the saturated solution inside a closed plastic (zip) bag with hygroscopic material in a different cup.
Now I'm looking for a different method to get faster and larger crystals... which seems to be rather difficult with NaCl (compared to CuSO4 for
example). |
Good suggestion! I think I may try coating my container with teflon spray. I want to use a wide size container, because I'm looking for a large lump
of crystalline material.
|
|
|