CycloRook
Hazard to Self
 
Posts: 89
Registered: 2-4-2018
Member Is Offline
Mood: No Mood
|
|
Grignard reaction products ?
I have attempted to do a grignard reaction multiple times.
My reaction calls to be dumped into a cool bath of saturated ammonium chloride solutions to hydrolyze everything.
I believe it dumped everything when it was to warm and I ended up with sludge that was hard to filter so I dumped everything in the hazmat bin.
I think I need to do this at a much lower temperature so the crystals are bigger.
Besides the grignard main product what are the side products and solids I'm removing ?
I'm guessing there is a ton of magnesium bromide and ammonium chloride but I don't know.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
im no organic expert but to give an answer i think most chemists would want some more detail, infact i think you might have to go to a psychic medium
forum to get accurate answers as we dont know what you initially put into the grignard
if youre serious about chemistry you have to acquire vacuum filtration system asap, they can be made somewhat cheap with an aspirator setup where you
shoot a water jet through the aspirator tube and this pressurized water going by will then pull a vacuum on the sidetube, otherwise a sturdy vacuum
pump is about 60 euros, 12 volt ones cost about 20-30 euros so you might as well throw a bit more into it at that.
if you mix the reaction mixture into ammonium chloride more slowly then yes precipitate should produce larger particles, you could maybe drip the
ammonium chloride solution into it
|
|
|
CycloRook
Hazard to Self
 
Posts: 89
Registered: 2-4-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Antiswat  | im no organic expert but to give an answer i think most chemists would want some more detail, infact i think you might have to go to a psychic medium
forum to get accurate answers as we dont know what you initially put into the grignard
if youre serious about chemistry you have to acquire vacuum filtration system asap, they can be made somewhat cheap with an aspirator setup where you
shoot a water jet through the aspirator tube and this pressurized water going by will then pull a vacuum on the sidetube, otherwise a sturdy vacuum
pump is about 60 euros, 12 volt ones cost about 20-30 euros so you might as well throw a bit more into it at that.
if you mix the reaction mixture into ammonium chloride more slowly then yes precipitate should produce larger particles, you could maybe drip the
ammonium chloride solution into it |
This is what I will do next time.
|
|
|
CycloRook
Hazard to Self
 
Posts: 89
Registered: 2-4-2018
Member Is Offline
Mood: No Mood
|
|
What happens to grignard reagent when you overheat it ?
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Cycloknight, sludges and emulsions can be maddeningly difficult to break.
Tell us what product you are shooting for, and we might be able to give you better tips.
Can you recover your product, simply by steam-distilling it out of your sludge?
|
|
|
CycloRook
Hazard to Self
 
Posts: 89
Registered: 2-4-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by zed  | Cycloknight, sludges and emulsions can be maddeningly difficult to break.
Tell us what product you are shooting for, and we might be able to give you better tips.
Can you recover your product, simply by steam-distilling it out of your sludge?
|
it's a variation of this reaction
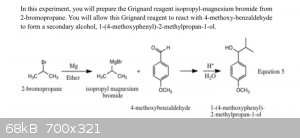
|
|
|
CycloRook
Hazard to Self
 
Posts: 89
Registered: 2-4-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by zed  | Cycloknight, sludges and emulsions can be maddeningly difficult to break.
Tell us what product you are shooting for, and we might be able to give you better tips.
Can you recover your product, simply by steam-distilling it out of your sludge?
|
I think I can. I just have no experience with steam distillation
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
You could try it. Be aware, there is a possibility of elimination. The product alcohol, could be dehydrated to an alkene, by heat.
Steam distillation only requires that your product be pretty insoluble in water, and that it has a reasonable vapor pressure at around 100 C.
You basically just put your reaction products in a big flask, add water, and distill. (Well, get the ether out first, if possible.)
Then, you keep adding water, and distilling, until your distillate starts coming over clear.
This could take a while. But, you end up with your water insoluble product, and water, in a two phase system. Just extract your now clean distillate
into a non-polar solvent. Evaporate off the solvent, and bingo!
|
|
|
Rainwater
National Hazard
   
Posts: 799
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
It sounds like a job for a dean stark trap. Heavy return mode
youtube
wiki
"You can't do that" - challenge accepted
|
|
|
medchemist
Harmless

Posts: 15
Registered: 10-7-2015
Member Is Offline
Mood: No Mood
|
|
have you tried adding a bit of HCl to see if that dissolves the Mg precipitate? You should then be able to extract your product out with ether or
ethyl acetate.
|
|
|
CycloRook
Hazard to Self
 
Posts: 89
Registered: 2-4-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by medchemist  | | have you tried adding a bit of HCl to see if that dissolves the Mg precipitate? You should then be able to extract your product out with ether or
ethyl acetate. |
That sounds amazing. Can someone please confirm this is the case. Would magnesium bromide
be attacked by HCL?
|
|
|
Bonee
Harmless

Posts: 29
Registered: 20-12-2010
Member Is Offline
Mood: No Mood
|
|
You need to keep in mind that one magnesium reacts with 2 halogen so the sludge you got is most likely some magnesium hydroxide, you need to add at
least one more eq. of dilute hcl (5-10%) to the cooled reaction mixture slowly until the rxn mixture is kinda clear or at least translucent.
If you have used DEE and not THF then you'll get a clear separation after the magnesium sludge is dissolved. If you've used THF you need to steam
distill it.
Can try to add some other immiscible solvent and do extractions but it will be a lot more work. (if the product is not steam volatile then it will be
a lot of work)
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 195
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
you need to add acid to dissolve the MgBrOH crap. As most precipitated hydroxides, it sucks to filter
|
|
|
Fantasma4500
International Hazard
    
Posts: 1677
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
if its hydroxides, in some cases adding excess NaOH can dissolve it
otherwise just add an excess of solvent and hope for it to seperate out, then you can decant off most of the solvent collecting - supposedly, an
amount of your product
weaker acids should work too, HCl may react with the product too
one could always try to shift the product back and forth between acid/base form in case thats doable
generally speaking solvents with a lower density, tends to allow precipitate to precipitate faster, density difference
if your precipitate has a density of 2.0 and your solvent has a density of 2.0, that could end up as a colloidal suspension
this is why sometimes it helps to add in a large amount of solvent when dealing with things that are very insoluble, as the soluble matter adds in a
bit extra density to the solution which can then make it take longer time to settle out
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Or you just do what was suggested before, add HCl, which won't react with the product. No need for excessive amounts of NaOH or solvent. No need to
wait for emulsions to separate. No need to do anything cumbersome you are talking about. Just a nice clear biphasic system you can separate in a
separatory funnel.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Or you just do what was suggested before, add HCl, which won't react with the product. No need for excessive amounts of NaOH or solvent. No need to
wait for emulsions to separate. No need to do anything cumbersome you are talking about. Just a nice clear biphasic system you can separate in a
separatory funnel or when not biphasic in the case of THF perfectly extractable.
|
|
|