twodogs
Harmless

Posts: 10
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
Benzaldehyde from Benzyl Chloride and Nitromethane
I found this but can't find any references for it. Has anyone seen this before and perhaps have a writeup?
Benzyl chloride can be converted into benzaldehyde by treatment with nitro methane and base. The reaction involves initial conversion of nitro methane
into its anion, followed by SN2 reaction of the anion with benzyl chloride and subsequent E2 reaction.
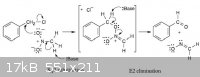
[Edited on 27-3-2011 by twodogs]
|
|
|
jwarr
Hazard to Self
 
Posts: 85
Registered: 25-6-2009
Member Is Offline
Mood: No Mood
|
|
a quick search on scifinder did not turn up anything for this reaction
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Where did you find it?
I weep at the sight of flaming acetic anhydride.
|
|
|
twodogs
Harmless

Posts: 10
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
I googled and found
http://www.solutioninn.com/chemistry/organic-chemistry/alcoh...
I am a bit dubious. I can't figure out how to balance the equation. Where does the chlorine end up?
[Edited on 27-3-2011 by twodogs]
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Im under the assumption that the Chlorine ends up with the base used but im confused as to what the final results of the nitromethane would be. Is
there some sort of resonance setup?
Im also having a bit of trouble understanding it but this is not one of my strong points in the first place. Im left to assume a 2x molar excess of
base being used as it seems to suggest to me that the Chlorine adds to the first mol of base while a second mol attacks the intermediat compound to
finish off the product.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
http://www.chemistry.esmartweb.com/nitro5.pdf 
Anions derived from aliphatic nitro compounds are capable of covalency formation at either
carbon or oxygen. Although the carbon alkylate is stable, and is isolated without difficulty, the
oxygen alkylate is unstable and the carbonyl compounds corresponding to the alkyl halides are
obtained. In general, nitroparaffin salts undergo oxygen alkylaton with little, if any, concomitant
carbon alkylation; indeed, this is the basis of a useful synthesis of aldehydes and ketones
(Scheme 5.1).1 However, there are some exceptions to give the C-alkylation, the reaction of
p-nitrobenzyl chloride with the salt of 2-nitropropane give the C-alkylation exclusively [see the
section of 7.1.1 radical substitution (SRN1)].
should work and there are reports from some of the guys on WD or now known as
the collective of it working from memory.
it is referenced too
Hass, H. B., and M. L. Bender. Org. Synth., ", 932 (1963).
ill dig it up and post it here if I can.
[Edited on 27-3-2011 by Ephoton]
e3500 console login: root
bash-2.05#
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
case closed 
Organic Syntheses, Coll. Vol. 4, p.932 (1963); Vol. 30, p.99 (1950).
o-TOLUALDEHYDE
Submitted by H. B. Hass and Myron L. Bender1.
Checked by Arthur C. Cope and Malcolm Chamberlain.
1. Procedure
Eleven and one-half grams (0.5 g. atom) of sodium is dissolved in 500 ml. of absolute ethanol in a 1-l. round-bottomed flask. Forty-six grams (0.52
mole) of 2-nitropropane is added, then 92.5 g. (0.50 mole) of o-xylyl bromide (Note 1). The flask is attached to a reflux condenser connected to a
drying tube and shaken at intervals for 4 hours. The reaction mixture, originally at room temperature, becomes warm spontaneously, and a white
precipitate of sodium bromide forms (Note 2).
After a reaction period of 4 hours the sodium bromide is separated by filtration and the ethanol is removed by distillation on a steam bath. The
residue of product and sodium bromide is dissolved in 100 ml. of ether and 150 ml. of water. The ether layer is washed with two 50-ml. portions of 10%
sodium hydroxide solution to remove any acetoxime and excess 2-nitropropane and is then washed with 50 ml. of water. The ether layer is separated and
is dried with 15 g. of anhydrous sodium sulfate, and the ether is removed by distillation on a steam bath.
The crude product is distilled from a Claisen flask under reduced pressure. The yield of o-tolualdehyde boiling at 68–72°/6 mm., n25D 1.5430, is
41–44 g. (68–73%) (Note 3).
2. Notes
1. o-Xylyl bromide may be obtained from the Eastman Kodak Company or may be prepared by the light-catalyzed bromination of o-xylene.2
2. The solution is originally supersaturated with the sodium salt of 2-nitropropane, and a precipitate of this salt may be mistaken for sodium
bromide.
3. This is a general method for the preparation of substituted benzaldehydes. The following aldehydes have been prepared by the same general
procedure.3
Aldehyde
Yield, %
p-Bromobenzaldehyde
75
Benzaldehyde
73
p-Carbomethoxybenzaldehyde
72
p-Cyanobenzaldehyde
70
p-Trifluoromethylbenzaldehyde
77
e3500 console login: root
bash-2.05#
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
I think you get a better yeild from hexamine though
e3500 console login: root
bash-2.05#
|
|
|
twodogs
Harmless

Posts: 10
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
Thanks for that.
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
no probs my friend I wouldnt post if but for your effort to forward the cause.
good luck with your search.
e3500 console login: root
bash-2.05#
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Ephoton  | case closed 
...
Eleven and one-half grams (0.5 g. atom) of sodium is dissolved in 500 ml. of absolute ethanol in a 1-l. round-bottomed flask. Forty-six grams (0.52
mole) of 2-nitropropane is added |
It says "2-nitropropane" which is not what the question was about. The oxidation of alkyl halides and sulfonates via the decomposition of alkyl
nitronates derived from 2-nitropropane is a common and well known method (there are plenty of references posted in other threads). But the original
poster asked about the reaction of benzyl chloride with nitromethane derived nitronate which is certainly neither common or well known.
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
true good point there nicodem I should have taken that into account.
case is open then till some one tries it.
the measurements should be the same and the theory and balancing of equations would
be the same as well.
e3500 console login: root
bash-2.05#
|
|
|
twodogs
Harmless

Posts: 10
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
So is sodium ethoxide or perhaps sodium methoxide necessary to create an anion. I was hoping simple NaOH would suffice.
[Edited on 29-3-2011 by twodogs]
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
I think sodium methyl nitronate is what is made when we make the big flame from nitromethane
and sodium hydroxide so I would think it should work.
I belive from my experiance with nitronates they use 2 nitro propane as it is not as reactive
when making the nitronate salt.
I could be wrong here but from what I have seen the larger the alkyl chain on the nitro the less likely
you are going to have the fire from hell burn your lab down.
still I have made the salt with nitromethane then done a neff reaction to formaldehyde so I can not
see how it would not work with normal sodium hydroxide.
I think they like to use ethoxide as it is in alcohol making the bromide precipitate.
also the reaction looks quite anhydrous from what I can tell so maby hydroxide is not so good.
maby if one was to make the nitronate salt first then dry it very carefully then use this in IPA as
this alcohol is easier to get anhydrous then add the benzyl chloride it might work.
e3500 console login: root
bash-2.05#
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
on second thoughts I think you would be better off just using sodium hydroxide ethanol
and toluene or xylene. then distill of azeotrope to form the ethoxide ready for use.
I dont think its the sodium hydroxide that would be a problem but rather the water.
this would save you the danger of isolating and drying the nitronate salt.
e3500 console login: root
bash-2.05#
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
It seems to be simple to do. All substrates are at hand: sodium, anh. ethanol, CH3NO2, benzyl chloride.
I am going to test this in a few days.
[Edited on 30-3-2011 by kmno4]
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Solution of sodium ethoxide has beeen prepared from 1,07 g Na and ~40 cm3 ethanol. Next I have added equimolar amount CH3NO2 in ethanol. I have got
white suspension, however liquid had blue tinge. Equimolar amount of benzyl chloride has been added to this suspension. The only effect is that liquid
has green tinge now. No reaction seems to take place, even after standing 12 h at room temp. I am going to heat to reflux this mixture tomorrow.
EDIT:
I had no time to reflux it. After 24 hours there is still white suspension but liquid is yellow now and misture starts to smell like isocyanide 
Something happens....
[Edited on 1-4-2011 by kmno4]
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Product of refluxing (5 hours) looks very shitty.
It is just brown, not transparent solution, with brown sediment (possibly contaminated NaCl) and isocyanide smell. It is not worth taking a picture,
the most adequate place for such things is water-closet.
But I will try to do "something" with it.
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
doesnt sound too good kmno4 thanx heaps for the test run.
I would have thought nitromethane to be more reactive than
the other nitroparifins.
nitroethane and nitropropane do not release anywere near
as much heat when mixed with dry NaOH as nitromethane.
[Edited on 4-4-2011 by Ephoton]
e3500 console login: root
bash-2.05#
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
I had no idea so I refluxed it additionally during ~12 hours.
(it is because some papers give procedures with 14 h refluxing and longer).
Isocyanide smell became weaker, but a new one - benzaldehyde - appeared. It all is not so simple because benzaldehyde can exist in this mixture as
benzaldoximes (in some papers they extract them with NaOH sol. from chlorofom sol. of reaction product).
However I think that this smell confirms existence of benzaldehyde (as one of the products of reaction).
It would be good is someone else would perform this experiment and present results here.
|
|
|
Slash
Harmless

Posts: 8
Registered: 2-4-2011
Member Is Offline
Mood: No Mood
|
|
This is very interesting kmno4! Keep up the good work i wish i could help
|
|
|
MeSynth
Hazard to Others
  
Posts: 107
Registered: 29-7-2011
Member Is Offline
Mood: http://www.youtube.com/watch?v=5ZltqlVuDIo
|
|
I found this in another forum... It needs to be revised.
Benzaldehyde:
140 g HMTA are finely grinded up and suspended into 4000 ml Ethanol (60%). To this suspension are added 125 g Benzyl Chloride, and the mixture is
refluxed with stirring on a water bath for 5-6 h. After the reaction is finished, there are added 2000 ml H2O, and the mix is transferred into a
distillation flask. After the alcohol distillated off, the stirrer speed was turned up, and the Benzaldehyde was steam distilled by the steam that
generates from the boiling water in the flask. The Benzaldehyde presents as a separate layer in the receiving flask and is very probably pure enough
to leave out further purification. (If desired, the BA can be extracted with DCM or ether, and can then be isolated as the Bisulfite adduct)
The yield of Benzaldehyde is 75 - 90 g (60 - 72%)
----------------------
another post clip
the sommellete I would do with just water. setup for reflux with water
then when the condensor does not smell like amine or chloride change
condensor for distil setup.
when distilling drip in water into reaction flask at the rate that water
comes over at. keep distilling till no organics come over.
sep distallate with dcm then dry with MgSO4, filter then.
evap dcm to get a very clean
aldehyde.
----------------------
another post clip
Just be sure to do a bisulfite workup to get rid of any remaining benzyl chloride.
|
|
|
MeSynth
Hazard to Others
  
Posts: 107
Registered: 29-7-2011
Member Is Offline
Mood: http://www.youtube.com/watch?v=5ZltqlVuDIo
|
|
I found a couple more... XD (these are by faaa the best I've read about)
Benzaldehyde from Benzal Chloride Using Hydrochloric acid.
[by Friedrich Bruhne and Karl-August Lipper : Krefeld Germany November 6, 1978]
322 g (2 mols) of benzyl chloride and 750 g of 25 percent strength hydrochloric acid are heated to the reflux tempurature in a 1 liter three-necked
flask with a stirrer, reflux condencer, gas inlet tube and thermometer, whilst stirring vigerously, and the mixture is kept under light reflux for 2
hours. A sump tempurature of 106* C. is established. A weak stream of nitrogen is passed through the flask during the reaction . The off-gas escaping
from the reflux condencer is absorbed in a washing tower, packed with Raschig rings, wish 600 g of water, which are circulated by means of a pump.
After cooling the mixture, 204 g of a light yellow coloured oil which, according to the titrimetric determination contains 98.1% of benzaldehyde
(=200.1 g pure benzaldehyde), are obtained as the organic phase. This corresponds to a yield of 94.3% of theory. The benzaldehyde contains 0.06% of
residue which cannot be distilled. *I skip the rest
Benzaldehyde from Benzal Chloride using Zinc Chloride
[by General Aniline New York, New York February 17, 1960]
To 700 parts by weight of distilled benzal chloride there was added 2 parts by weight of anhydrous zinc chloride and the two were mixed thoroughly
with stirring while heating to 105—110°C. As soon as the reaction mixture was at 105—110°C. the addition of water was begun at a slow rate,
while maintaining the reaction temperature at 110—120°C. The addition of water was continued until a total of 85 parts by weight of water had been
added (approximately 5% excess) at which time the evolution of hydrogen chloride ceased. Reaction temperature was maintained for another hour to be
sure that all the HC1 had been removed, and the benzaldehyde was then removed by vacuum distillation. The yield of benzaldehyde recovered at non
distillation was 95% of theory and the purity of the product better than 96.5% Source General Aniline 1962
Manufacture of Benzaldehyde from Benzyl Chloride using Hexamine
[by Fabriques Issy, France March 7, 1912]
A solution of 14 kilos of hexamethylenetetramine in 40 litres of alcohol of 60 per cent. strength is mixed with 12.5 kilos of benzyl chloride and the
mixture is heated on then water bath in a reflux apparatus for 5-6 hours; 20 litres of water are then added, the greater part of the alcohol distilled
away and the residue carried over with steam. The liquid carried over is extracted by means of a volatile solvent and the aldehyde is purified by
means of its bisulphite compound. The yield of purified benzaldehyde amounts to 7.5 to 9 kilos. The reaction may also be carried out without addition
of alcohol,
that is to say in an aqueous solution. Thus, 12.65 kilos of benzyl chloride are heated for two hours with a solution of 14 kilos of
hexamethylenetetramine in 60 litres of water, and the aldehyde is then distilled with steam; after purification with sodium bisulphite a good yield of
benzaldehyde is obtained. Source: Fabriques 1914
|
|
|
2bfrank
Harmless

Posts: 5
Registered: 11-9-2009
Member Is Offline
Mood: No Mood
|
|
The initial reaction in this thread was a question in the prescribed text, namely McMurray 6E/ i.e. Describe the mechanism when the base produced
anion of nitromethane reacts with benzyl chloride. This tells me its in the literature. Im on an interem spell from Uni, and lost access to the
searching journal capabilities, till I return for more punishment, but Im sure that its out and waiting to be discovered. I might play around with
reaction conditions myself.
The most intelligent statements can be said so that an average person can understand, hence all the statements that require so much effort trying to
be understood, are an indicator that such a statement is not about presenting something of interest or intelligence, but rather an appearance of being
so.
|
|
|