Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Michler's Ketone
MIchler's Ketone is a well known component of several dyes and its preparation is described in several books on dye chemistry, eg Processes in dye
chemistry by Fierz-David & Blangey (SM library).
It is prepared by reacting carbonyl chloride (phosgene) with dimethylaniline... and there's the problem... phosgene. OK on an industrial plant but not
exactly amateur friendly.
Does anyone know of an alternative route? I was wondering if it is possibly to oxidize the appropriate bis(dimethylaminophenyl)methane which is
readily prepared from formaldehyde and dimethylaniline? The oxidation of tetrabase, the tetramethyldiaminodiphenylmethane, to the analogous hydrol
with lead dioxide is described in Fierz-David's book too so in theory it should be possible to oxidise it a little further to the ketone.
Anyone any ideas?
[Edited on 16-8-2022 by Boffis]
|
|
|
digga
Harmless

Posts: 39
Registered: 11-6-2018
Member Is Offline
|
|
https://patents.google.com/patent/US2231067A/en
|
|
|
Lionel Spanner
Hazard to Others
  
Posts: 163
Registered: 14-12-2021
Location: near Barnsley, UK
Member Is Offline
|
|
Carbonyl diimidazole might work as a substitute for phosgene, though it's less reactive.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2656
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Here are a variety of ways to make it, which include the oxidation of the methane to ketone, but there are a variety of ways to do it.
Sadly, while CDI is great for many things, it cannot do the Freidel-Crafts chemistry needed. Triphosgene would likely work, but also hard to find.
Attachment: MIchlersKetonePreps.pdf (387kB)
This file has been downloaded 182 times
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Many thanks guys for the responses! My god Bob, some of those method in your scifinder download look complex, particularly the oxidation of the hydrol
to ketone route, is there any particular theoretical reason why they need such obscure oxidizing agents/conditions? I think I might just try a few
more accessible oxidizing agent first.
Also could there be a route through say dimethylaminobenzoic acid, perhaps via its acid chloride and a Friedel Craft type condensation with
dimethylaniline? This then raises the question of how to introduce the carboxylic acid group into the 4 position. I have recently recovered some
4-nitro dimethylaniline from another experiment so I could probably reduce it to the amine, then via the Sandmeyer route to the nitrile and hydrolysis
to the acid. Give the ease with which dimethylaniline undergoes substitution in the 4 position are there any more direct routes that any one can think
of?
Two other routes have just occurred to me. Does anyone think that the methylene group is reactive enough to react with either a nitroso group (say
nitrosodimethylaniline) or an alkyl nitrite to give the appropriate imine or oxime.
The second is the possibility of oxidizing the methylene group with sulphur to the thioketone and then de-sulphurisation as per the scifinder
references give in Dr. Bob's post.
[Edited on 19-8-2022 by Boffis]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I have just spotted an interesting compound in relation to the preparation of Michler' ketone, the dye Auramine O:
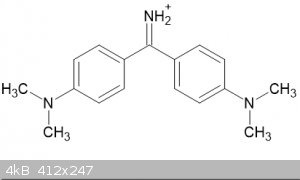
i was wondering if it would be possible to react the dye, which is usually solid as the chloride, with sodium nitrite and then heat the resulting
auramine nitrite? Would it decompose like ammonium ions do into nitrogen and in this case the ketone?
|
|
|
SplendidAcylation
Hazard to Others
  
Posts: 196
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
Quote: Originally posted by Boffis  | I have just spotted an interesting compound in relation to the preparation of Michler' ketone, the dye Auramine O:
i was wondering if it would be possible to react the dye, which is usually solid as the chloride, with sodium nitrite and then heat the resulting
auramine nitrite? Would it decompose like ammonium ions do into nitrogen and in this case the ketone? |
Ooo that's interesting!
I'm not sure why you suggested sodium nitrite, because I'm not really familiar with the reactions of nitrites, so maybe I'm missing something obvious,
but isn't that auramine dye just a protonated imine?
The hydrolysis of an imine to a ketone, as far as I know, is really simple, just reflux it with acid, I think?
The hydrolysis of an imine to a ketone is one of the steps in the synthesis of ketones by Grignard reaction that I did recently, it just involved
adding acid and refluxing.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
My expectation would be that, due to sterics, dimethylaniline primarily reacts at para.
For example, the reaction of dimethylaniline with formaldehyde gives primary bis-(4-dimethylaminophenyl)-methane:
https://pubs.acs.org/doi/10.1021/ja01160a008
The product may be amenable to oxidation at the methylene linker.
As for the 4,4'-bis-dimethylamino-benzophenone imine, I believe this can be hydrolyzed directly with an appropriate acid catalyst. Some papers mention
silica gel.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|