Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Vacuum fractional distillation
Vacuum fractional distillation is used to seperate compounds with close boiling points that would otherwise be difficult/impossible to distill with
normal distillation setup.
There is a lot of information about vacuum distillation on the web, but what I am trying to find out is when specifically it can be used?
Take nutmeg for example, it contains several fractions--
Could they be isolated?
Above all, I'm trying to understand when, and when not, vacuum distillation is utilized.
In the picture is that a air bleed tube going into the distillation flask?
[Edited on 11/2/2022 by Yttrium2]
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
And is vacuum distillation done by cranking up the heat on the hotplate and then watching for spikes/plateaus on the thermometer, before turning the
cow?
Did someone previously state that the vacuum levels cannot be adjusted?
Would I even need a cow?? Couldn't everything that boils off below the desired temperature simply be discarded?
[Edited on 11/2/2022 by Yttrium2]

|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Is it as simple as collecting the right fractions-- the one with the desired boiling point??
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
What would be a good vacuum distillation experiment?
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
I guess one could not discard the stuff that comes over first without breaking the vacuum.
So the cow is likely essential.
|
|
|
Chemgineer
Hazard to Others
  
Posts: 174
Registered: 25-5-2021
Member Is Offline
|
|
I suppose you could look up the boiling temperature vs pressure graphs for various liquids and if the gap between temperatures increases as the
pressure reduces then vacuum will help you.
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Cow is not essential. One method is to break the vacuum between fractions. This likely means you change the temp/pressure conditions between
fractions as well, which might be advantageous.
However, big changes to P/T conditions in the middle or a process are something to generally be avoided. In a normal setup, P is kept constant and
the temperature at the still head informs you as to what is happening in the system. You lose this information if you break the vacuum.
There is much to learn about simple distillation procedures before adding the complexity of a vacuum. Unless there is a particular need, I would be
sticking with atmospheric pressure for most situations.
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
When would it be beneficial to add a vigreux?
I think a short path vacuum distillation would be more suitable for seperating the compounds in essential oil, would it?
Also, is ever a reflux column (vigreux) added to short path distillation?
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Depends on which compounds in which oil.
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
Wouldn't the pear shape flask prevent magnetic stirring?
Both devices are considered short path distillation.
In which instance is the micro short path distillation apparatus used?
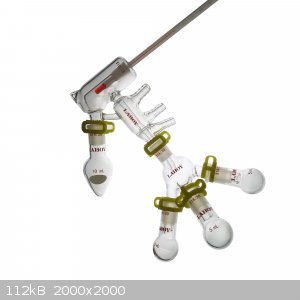
[Edited on 11/3/2022 by Yttrium2]
|
|
|
violet sin
International Hazard
    
Posts: 1475
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
Quick look has nutmeg oil "approximately 90% terpene hydrocarbons" as listed there have BP around 150-180°C ... The safrole you want is in the
232-234°C BP area. Wiki didn't have BP for the other components of phenolic character listed.
I'd look closely at what would be trying to come over with it. Price of nutmeg oil with 90%+ losses for small amounts of recreational fun sounds like
an overall failure economically. Personally I'd just not do that.
Your goingt o have to make a list of what's there, what easily leaves, what your left with. Does 170°C damage anything by default. Might have to
use vacuum any way. But more gaming it out needed yo. I'd love to see a sheet of paper littered with effort in your handwriting. Think you'd answer
many of your questions with an organized attack on the subject. Keep a binder, book or note pad and try to work it out.
Perhaps I'm the only one that feels this way? But you would learn more doing the work. Otherwise just be a consumer and enjoy someone else's
reliable time and effort with consumables
-VS
As always , not trying to be an ass or hurt feelings. Best of luck
|
|
|