Chemist1357
Harmless

Posts: 29
Registered: 26-10-2022
Member Is Offline
|
|
Bromination of 1,3 benzodioxole to 5-Bromo-1,3-benzodioxole?
Any way to do this without NBS that is more available to the amateur?
Could it be done with Using Ammonium Bromide and H2O2 in Acetic Acid?
Ammonium bromide is easily prepared using HBr and ammonia.
(Taken from Erowid) https://erowid.org/archive/rhodium/chemistry/aromatic.bromin...
Experimental
General Procedure for the Bromination of Anilines and Anisoles
A 25 mL two-necked round bottomed flask was charged with the substrate (2 mmol) and ammonium bromide (2.2 mmol) in acetic acid (4 mL). A 30% H2O2 (2.2
mmol) was added dropwise to the reaction mixture and the contents allowed to stir at room temperature. The reaction was monitored by thin layer
chromatography (TLC). After the completion of the reaction, the reaction mixture was treated with saturated sodium bicarbonate solution and extracted
with dichloromethane. The organic extract was dried over anhydrous sodium sulfate and solvent evaporated under reduced pressure.
This could be done to 1,3 benzodioxole correct? Or will you end up with something else...
[Edited on 27-11-2022 by Chemist1357]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Table 2, entry 8.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
_not_Atara, clearly you have a wicked sense of humor. 
But you were 100% honest, I checked.
Table 2, entry 8 tells everything.
Unfortunately it is not the wanted isomer, but that's life and organic chemistry in action.
|
|
|
Chemist1357
Harmless

Posts: 29
Registered: 26-10-2022
Member Is Offline
|
|
Quote: Originally posted by Pumukli  | _not_Atara, clearly you have a wicked sense of humor. 
But you were 100% honest, I checked.
Table 2, entry 8 tells everything.
Unfortunately it is not the wanted isomer, but that's life and organic chemistry in action. |
How is it not the wanted isomer? What is the obtained product then?
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
You put a question mark to the end of your opening sentence, hence I assumed you were looking for a route to 5-bromo-isomer. (ie: your wanted isomer
was the 5-bromo-compound.)
Table 2, entry 8 says the product is exclusively the 3-bromo-isomer.
If you were after the 3-bromo, that's fine, you can get it in good yield from the reaction. If you wanted to produce the 5-bromo then the described
route is obviously not the proper one.
Btw. I think the 5-bromo-numbering is not good, not IUPAC compatible at least. 3-bromo: the bromine goes to the first carbons that are the immediate
neighbours of those that have the O attached. 4-bromo: the bromine goes to the second carbon from the O-bearing one. 5-bromo: I can't understand this,
due to the symmetry of the molecule.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Pumukli: There is a footnote signifier by the entry, but the footnote is obviously wrong, because it says something about a naphthalene (impossible).
Should download the actual paper to check.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Atara, (or not), thanks for the reminder!
Here we go:
Attachment: krishnamohan2004.pdf (152kB)
This file has been downloaded 193 times
It seems, that this table is a bit different than on the rhodium site.  And the
mystery of "naphthalene" also got solved. And the
mystery of "naphthalene" also got solved. 
[Edited on 29-11-2022 by Pumukli]
[Edited on 29-11-2022 by Pumukli]
|
|
|
Boffis
International Hazard
    
Posts: 1836
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi Pumikli, I think the difference in the numbering is due to the fact it is regarded as a benzo-heterocyclic rather than a benzene derivative so the
numbering goes like this:
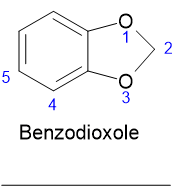
Like you say there seems to be a fairly fundamental difference between the Rhodium table and the original paper. I presume that, as you say, the "p"
isomer is the 5 isomer and the "o" isomer is position 4
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Yeah, you are right, Boffis, my fault! 
Anyway, the "real deal" table shows a very different picture!
|
|
|
Mateo_swe
National Hazard
   
Posts: 505
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
Couldnt just liquid bromine be used, or would that put the bromine in wrong position?
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Maybe the orientation would be different.
Maybe the availability is different (for an amateur).
|
|
|
Chemist1357
Harmless

Posts: 29
Registered: 26-10-2022
Member Is Offline
|
|
In acetic acid yes yields are around 70% and you get the dibromide as well.
Also screw working with Br2 when you could use a much nicer bromide salt such as ammonium bromide (i'm hoping)
[Edited on 29-11-2022 by Chemist1357]
|
|
|
Mateo_swe
National Hazard
   
Posts: 505
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
Now i remember, what i was refering to.
It was a bromination of protocatehuic aldehyde (3,4-Dihydroxybenzaldehyde).
That aldehyde can be brominated with bromine in chloroform resulting in 2,5-dibromo-3,4-dihydroxybenzaldehyde.
Not the same as 1,3-Benzodioxole obviously, i was just remembering wrong and got them mixed up.
Sorry about that.
[Edited on 2022-11-29 by Mateo_swe]
|
|
|
Johnny Windchimes
Hazard to Self
 
Posts: 61
Registered: 28-5-2019
Member Is Offline
Mood: Sorry, it's my chimes~!
|
|
I got what you need son....
Truly the best way IMO.
If you cant find HBr, make it from NaBr.
If you cant find 30% H2O2, make it by slowly and carefully boiling down regular 3% H2O2 until it's to your desired concentration.
BE WARNED: YOU MUST vacuum distill the final product; it'll scorch and turn to tar otherwise.
It's worth it, even if you have to make the hydrobromic acid, it's easy if not tedious. I'll never use elemental bromine or NBS or anything like that
again!
Good luck~!
Attachment: Dat 5-Bromo Narc Paper - Copy.pdf (1.8MB)
This file has been downloaded 166 times 
P.S. Not dead, just been busy.... for over a year....
[Edited on 20-12-2022 by Johnny Windchimes]
~Incredibly profound and/or wise quote goes here~
|
|
|