| Pages:
1
2
3 |
Hey Buddy
Hazard to Others
  
Posts: 384
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
NIHT/NHHT Synth Suxess!
Nitroguanidine and Hexamine can be fragment-cyclized to 2-Nitriminohexahydro-1,3,5-triazine monhydrochloride (referred to in lit. as NHHT or NIHT)
with hardware store muriatic acid under mild conditions and basic equipment.
NHHT is reported (without verification) to detonate at 9000m/s velocity with a density of 1.95g/cc (Yongzhong, Synthesis of Polynitrocompounds from
Nitroguanidine, propellants, Explosives, Pyrotechnics 14, 150-152 [1989]).
It's futrther reported that the compound "explodes" on heating to 102 C. Other literature claims m.p. 189 C without mention of explosion.
This material came to attention during the failure to synthesize NTNT "new melt cast explosive", as a possible side reaction. Preparation of this
compound was attempted by literature procedures by (me)hey buddy, repeatedly, without success. The reported high performance, simple preparation and
ionic nature of this compound make it very unique in its class as a triazine, and its preparation would be beneficial to the arsenal of explosive
material enthusiasts. As a compound, NHHT is regarded in the defense industry highly, and is "compared" with RDX and CL-14 as an approximate in that
class. Whether this comparison is realistic or not remains unknown as this compound somewhat unfamiliar to explosive researchers, but because of its
ease of preparation, should be.

[Edited on 8-12-2022 by Hey Buddy]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 384
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Successful Procedure
Successful rendering of NHHT was not precipitated by Metelkina's literature procedure in "2-Nitroguanidine Derivatives: X Synthesis and Nitration of
4-Nitrininotetrahydro-1,3,5-oxadiazine and 2-Nitrinino-1,3,5-triazine and their Substituted Derivatives". This procedure in my attempts failed
repeatedly. At some point in time I found an old file saved on my computer titled "NIHT" which is referring to the same compound by different acronym.
I have no memory of where this file came from. It is most certainly from a thread on SM from the past, but I cant find it. This procedure is in the
attached picture. This procedure was successful scaled down, in many variations. The primary factor of success is closed vessel and long term reaction
time.
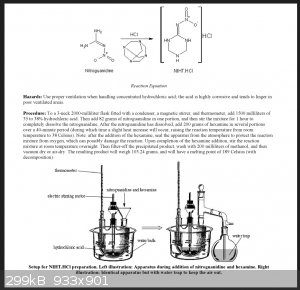
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 384
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Scaled down version:
150ml HCl (sunny side, muriatic)
8.2 g Nitroguanidine (NQ)
20 g Hexamine (Hx)
No cooling is employed in this reaction and it takes place at ambient temperature with exothermic response upon addition of hexamine.
Reaction is carried out in a 1000 ml boiling flask. Teflon tape is stretched across the opening of the boiling flask which allows the flask to off-gas
but refuses entrance of air which can affect yield. The teflon tape was secured by wrapping it with some electrical tape around the boiling flask
neck. This tape remained in place the entire 8 Hours of reaction to prevent ingress of air.
150 ml HCl is added into reaction vessel.
8.2 g NQ is added and stirred until entirely dissolved at room temperature.
20 g Hx is added to reaction vessel in one portion, stirring continues
Reaction vessel is closed from ingress of atmospheric air
Reaction is continued with stirring and without cooling for 8 Hours
Reaction media becomes banana colored and opaque
At conclusion of 8 hours, 500 ml dH2O is added to reaction to quench it
White opaque solution is precipitated
Filter and wash with dH20
NHHT*HCl is ionic and may have application as a high energy ligand in coordinated explosive complexes such as prospective complex: e.g.
[M(NHHT)x(ClO4)y] / [M(NHHT)x(NO3)y]
   
[Edited on 8-12-2022 by Hey Buddy]
|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
That is very nice. I had upload that file in the past, you can find it at the "trying for keto RDX" together with some other files. How much was your
final yields ? Will you convert it to nitrate salt, then use H2SO4 for the dinitrate (NNHT) ?
[Edited on 8-12-2022 by underground]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1335
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
It seems easy.....And the substance on filter is explosive, or only precursor ? I readed all twice. But is it not clear for me. Thanks.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
It is not. It is the HCL salt. The nitrate/perchlorate salt should be though.
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 384
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Sorry to be unclear. It is confusing researching the compound because there are contradictory statements in literature, and multiple acronyms. This
method prepares the HCl salt. According to literature, the HCl salt detonates at 9000m/s. This is unverified and I can find little cross reference
material supporting this. NHHT when nitrated with H2SO4 or NH4Cl produces NNHT which has a nitramine group at the number 5 triazine position. NNHT is
considered a high energy insensitive secondary explosive.
The HCl salt has been of interest to me due to possible decomposition at 102 C as is claimed in literature, which in the case of "NTNT" was a concern.
It appears this claim is incorrect about an explosive decomp at 102 C. My further interest in this material is the prospect of preparation of basic
perovskite explosive complexes from NHHT as an easy ligand, because of its ability to form salts. The character of the NHHT*HCl is unknown and may be
misreported where found. To the best of my understanding now, the HCl salt is still very high velocity in detonation and high density. I have not
begun testing on the material yet, the first sample is drying and I prepared a 2x scale sample that is on filter right now. Theoretically, NHHT*HCl
should serve as a high energy ligand in a perchlorate or nitrate complex and its sensitivity should be somewhat adjustable by experimenting with the
oxidizer and metal of the complex.
I will add a yield when the first batch is dry. I can see now it is low yield, I think extending the reaction time will increase yield. Any yield at
all is a good first step because I have failed to yield anything so many times in attempt of NHHT HCl. The second batch was left to react longer and I
will post that info too when dry.
EDIT: I dried some on a pan at ~44 C but I didn't move the material around while drying, just left it on the pan, some of it turned yellow but appears
not to be a major decomposition. 6 g was the total yield, which is lower than expected and is probably due to 8 hour reaction time 12 hours of
reaction would probably yield more product. I would also recommend a larger washing with water bath at end of reaction to make less acidic.
[Edited on 8-12-2022 by Hey Buddy]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1335
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
Thanks. On paper is acidic salt of HCl. Is it explosive with low sensitivity (or unknown sensitivity).
Therefore is impossible do it neutralisation for example baking soda. Or using else alkaline neutralisation. (NaOH, KOH, NH4OH) ...?... this salt
(NNHTxHCl) can react with HClO4...?...for create some better material? Thanks.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 384
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Quote: Originally posted by Laboratory of Liptakov  |
Therefore is impossible do it neutralisation for example baking soda. Or using else alkaline neutralisation. (NaOH, KOH, NH4OH) ...?... this salt
(NNHTxHCl) can react with HClO4...?...for create some better material? Thanks. |
Should be able to use acid or double replacement reaction to change the anion with any negatively charged one like CLO4. It should be able to be
neutralized relulting in the free base. It should be able to create lots of materials with different properties. The HCl should already be energetic.
I will test hammer and burn test on the regular HCl salt and then try a Cu and Ni salt. I will do this with the guanidine thread also and post result.
I will not be treating the NHHT with HNO3/HClO4 because my interest is to push away from these in reliance and simply learn metathetical reactions
with acid salts to reduce reagents.
|
|
|
Microtek
National Hazard
   
Posts: 827
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
The question is how labile such salts might be. I found that aminonitroguanidine nitrate loses nitric acid over time due to being a quite weak base.
Seeing that NHHT*HCl is reportedly freebased by treating with hot 18% HCl, NHHT must be an exceedingly weak base.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1335
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
HCl salt, preparation by description above. Run was 8 hours stirring at 17 Celsia. + 8 hours without stirring at 22 Celsia. . After this at 20 C no
any precipitat in yellow solution. After cooling on + 5C arise precipitate. Vacuum filtered. HCl salt has not any energetic properties. Nothing
burning, no flame on alu foil. Only smoke and smell similar as hexamine. Big hammer test repeatedly, but without any explo.


Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
I was not expecting the HCL salt to be energetic. What was your yields? Try the nitrate/perchlorate salt. The nitrate salt probably can be treated
with H2SO4 to form NNHT.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1335
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
Nitrate/ perchlorate from HCL salt will examinate later. Within some days. Yield will measure also later. After dry process.
[Edited on 9-12-2022 by Laboratory of Liptakov]
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite (2024)
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 384
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Quote: Originally posted by Laboratory of Liptakov  | HCl salt has not any energetic properties. Nothing burning, no flame on alu foil. Only smoke and smell similar as hexamine. Big hammer test
repeatedly, but without any explo.
|
I made an error. The Yongzhong P.E.P. article clearly claims that the HCl salt is Vd 9000m/s, explodes at 102 C. This misinformation has caused a lot
of confusion for me. Maybe they mixed up their molecules?-- The comparison to CL-14 is to NNHT not NHHT as I suggested. I went back and found the NNHT
power point where that comparison is made and realized my mistake, sorry about that.
Darn. Was really hoping the literature was accurate on this molecule being energetic. I tried a Cu ClO4 * NHHT --by CuSO4*5H2O + NH4ClO4 and had no
precipitation. Tried isopropyl extraction that sometimes works with other combinations and got no crystals... I tried adding another ligand to see if
it would co-precipitate. Now I just have a jar full of random non explsive things.
I made a second batch of NHHT*HCl, I will test it on big hammer and burn to double verify.
If the HCl salt is not energetic, but you do want it to be energetic, if you nitrate it in H2SO4 it should form an energetic compound. NNHT. Here is a
defense industry power point on NNHT.
Attachment: Nnht.pdf (368kB)
This file has been downloaded 165 times
Microtek, I'm sure you're right. I'm unfamiliar with coordination chemistry. --This is off topic, but Microtek, do you think "Allantoin" could be
interesting in typical perovskite coordination ie. allantoin metal perchlorate? I found some affordable as a womens cosmetic supply, so I ordered a
bag of it.
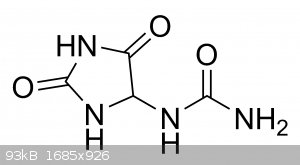
[Edited on 10-12-2022 by Hey Buddy]
cause it looks kind of interesting to me... im curious, anyways...
[Edited on 10-12-2022 by Hey Buddy]
|
|
|
Microtek
National Hazard
   
Posts: 827
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I can't say that I'm any kind of expert in that area myself, but I agree that the molecule has the look of something that could be both dense and
energetic if a few nitro groups were introduced. You could probably also just make a perchlorate or nitrate salt (searching on the web yields a few
references to the nitrate but none that I can find to the perchlorate), but I think you need to experiment to establish the stability, hygroscopicity,
etc.
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 384
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
I have finally gotten around to double-verifying NHHT is not energetic to burn nor hammer. Too bad. NHHT salts/complexes seem like the next step in
exploration.
|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
I just spend few minutes to draw the derivatives form NQ/Urea + Hexamine
Also the Dinitrate/Diperch salts may also exist from the low nitrated derivatives
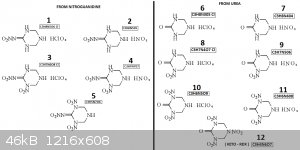
[Edited on 10-7-2023 by underground]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 384
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
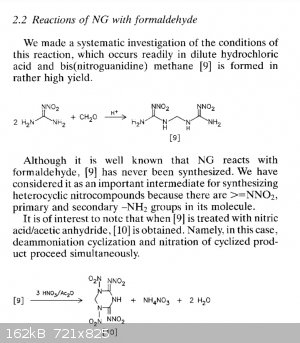
The general theme of this thread was NHHT, but in the hopes of something more powerful from nitroguanidine. Here are some related compounds from
nitroguanidine. The second paper has some (bis)nitroguanidine methane heterocycles that look pretty interesting and simple.
These images are all from the second polynitro paper. They report the open molecule [7] to be 1.93g/cc but have Vd @ 1.83g/cc of 9020 m/s but with a
decomp only slightly over 100 C. Not sure if the hexamine/NQ/HNO3/H2SO4 would be more powerful, but you can take that molecule and treat it with NaOH
then free it from the sodium to get [7]. Thats according to the article. Or you can use ammonia and leave the NH4 as cation. Hydrazine would be
interesting.
The most interesting is the bis nitroguanidine [9], and molecule [11] which is a sort of dinitrimino heterocycle. Theres also [15] which is an
oxadiazine, I have no idea how that would perform but it is interesting with the oxygen in the frame of the molecule.
Attachment: nitroguanidine derivatives.pdf (134kB)
This file has been downloaded 101 times
Attachment: nitroguanidine poly nitro compounds Zhuang.pdf (283kB)
This file has been downloaded 110 times


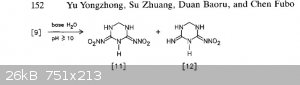
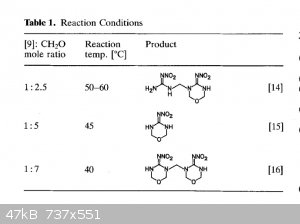
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 384
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Quote: Originally posted by underground  | I just spend few minutes to draw the derivatives form NQ/Urea + Hexamine
Also the Dinitrate/Diperch salts may also exist from the low nitrated derivatives
[Edited on 10-7-2023 by underground] |
IIRC, microtek didn't think number 1 and 2 would be good candidates for further salts, although I didn't attempt it either way, I do have some NHHT
sitting around... I sort of lost interest when I realized NHHT didn't explode on heat as claimed in a paper.
Did LL ever get aaround to testing NHHT nitrate perchlorates? He mentioned he might try that. Check the polynitro paper for [3] which is the product
of mixed acid treatment. Probably would work with nitrate salt/H2SO4/. It is more realistic than any of the higher nitros requiring Ac2O. I suppose
its possible the higher nitros such as 'z' rdx compound [2] may possibly be accessible without Ac2O via hexamine dinitrate route.
[Edited on 10-7-2023 by Hey Buddy]
[Edited on 10-7-2023 by Hey Buddy]
|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
The NHHT nitrate and perchlorate seems the most atractive to me since they would be dead easy to make
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 384
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Thats true. I have the HCl right now. Not sure the best bet to freebase? I could try NO3. Could also attempt NH4ClO4 driven displacement. Ive got a
few grams of NHHT sitting around...
Do you think 3 and 4 are even possible? It seems unlikely to me.
[Edited on 10-7-2023 by Hey Buddy]
|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
To remove the hcl from NHHT you have to add a stronger base like NaOH in order to absorb the HCL . NHHT freebase will yield.
Another way is to dissolve NHHT HCL and silver/lead nitrate/perch at equal molarity. Lead hcl and Silver hcl will ppt out since they are almost
insoluble in water. NHHT HNO3/HCLO4 will remain dissolved in solution
NHHT Nitrate added to Concentrated H2so4 most likely will yield 3-4 freebase.
The perchlorate salt ( 3 ) may exist since perchloric acid is a very strong acid. Not sure about 4
NHHT HCL molar mass:
C3H8N5O2Cl -->12.011*3+1*8+14*5+16*2+35.453= 36,033+8+70+32+35,453= 181,5 g/mol
NaOH molar mass is 40g/mol
So for every 181.5g of NHHT HCL you have to add 40g of NaOH to freebase it
Note that NHHT freebase may still be soluble in water. If that is the case then you have to evaporate all the water so you will end up with a mixture
of NHHT and NaCL. Then extract the NHHT with an organic solvent.
The silver nitrate route would be the best though IMO
HCLO4 acid is stronger than HCL acid so adding an equal molar mass of NHHT HCL and HCLO4 will yield NHHT HCLO4. Upon evaporation HCL acid will go off
[Edited on 11-7-2023 by underground]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 384
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Ok AgNO3 metathesis it is then.
It worked okay. There is a bit of silver nitrate in filtrate. The AgCl was totally insoluble. I heated it up a bit to increase nitrate solubility for
separation. Evaporating filtrate now...
[Edited on 11-7-2023 by Hey Buddy]
|
|
|
underground
National Hazard
   
Posts: 694
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Nice. Next step is to check its properties. The perchlorate salt has to be tested too. If you dont mind keep a little bit to ckeck if with H2SO4 will
react at low temp (0c) to yield the 3/4 freebase (NNHT). 3/4 could also be tested and checked if the nitrate/perchlorate salt is possible.
Also the dinitratediperchlorate salt of NHHT may be possible.
This can be tested by reacting NHHT Nitrate with equal molar mass of 65% HNO3
[Edited on 11-7-2023 by underground]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 384
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Quote: Originally posted by underground  | Nice. Next step is to check its properties. The perchlorate salt has to be tested too. If you dont mind keep a little bit to ckeck if with H2SO4 will
react at low temp (0c) to yield the 3/4 freebase (NNHT). 3/4 could also be tested and checked if the nitrate/perchlorate salt is possible.
Also the dinitratediperchlorate salt of NHHT may be possible.
This can be tested by reacting NHHT Nitrate with equal molar mass of 65% HNO3
[Edited on 11-7-2023 by underground] |
"3/4" should come out as compound [3] (from polynitro paper). I dont think they can continue to hold the anions after the second nitro is added.
I suspect "5" is possible via HDN + NQ + HCl. If that were correct, 5 may have good performance with few steps. Is it possible to produce HDN without
65% NA? For instance a nitrate salt? If that were possible it would be very simple to produce "5". Z rdx decomposes at 50C which is Impractical but
the nitimino with 2 nitrimines could Be much more thermally stable...
There were 2 g of NHHT converted to the nitrate, still have around 4g NHHT HCl remaining. If any of it shows promise I can whip up so more, i's pretty
painless. I used quite a bit of water so it is still evaporating but I looked at it this morning and much of it has dropped out of solution already.
The solution turned a tint of purple. I assume it is from reverted Ag in solution.
For NHHT nitrate, I think perhaps fire it into an Al block and dehydrate some with H2SO4 then fire that into Al for comparison? What mass of load is
the standard for firing into Al cubes for comparisons, half gram?
Guess I need to prepare HDN to test if produces 5 with NQ...
[Edited on 11-7-2023 by Hey Buddy]
[Edited on 11-7-2023 by Hey Buddy]
|
|
|
| Pages:
1
2
3 |