| Pages:
1
2 |
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
Warfarin syntheses
Background
A couple of years ago, an EU directive imposed licensing on all use of pesticides, among them rat poisons. In my country, this resulted in one
dominant pest control company almost holding a monopoly on rat elimination for businesses as well as for private individuals.
And what do companies do whenever they're alone on stage? They raise prices. The cost of having someone come over and place little boxes of
anti-coagulant poison in the crawl space under your house soon doubled, then tripled. It now costs well over €1000 for a first visit, and if you
want the pest control to continue, you're forced into buying a subscription to their services ... you get the idea. Net cost can land you well above
€2000.
This calls for rebellion.
The most widely used rat poison in my country is warfarin, an anti-coagulant also widely used by humans as an anti thrombotic
medicine for a wide variety of medical conditions. A blood thinner. Ii works by blocking the recirculation of vitamin K in the body (vit K is needed
as a cofactor for certain key enzymatic pathways in the coagulative activity in the blood).
It's used as a rat poison (among many other substances in the same family of molecules, 4-hydroxycoumarin derivatives) because of its delayed but
definitive action. If the rat drops dead next to, or too close to the poison, other rats are smart enough to avoid it. But the internal bleedings
caused by warfarin will not be fatal until a day or two later.
It's an attractive solution. Any accidental poisoning of pets, children or neighbours can easily be reversed by vitamin K injections, and managing
warfarin intoxications is routine business for health care givers. Not long ago every other Little Old Lady was medicated with warfarin for their
atrial fibrillations. And oh did they overdose.
So, warfarin. Let's go!
Aim of this project
To find a realistic way for the Mad Home Scientist to synthesise his/her own warfarin for rat control using reagents that are cheap and available.
introduction
First off, a search of this site.
Wiki article: https://www.sciencemadness.org/smwiki/index.php/Warfarin
Doesn't offer much in ways of guidance that one cannot get from regular old wikipedia.
I also found this A thread describing the synthesis of coumarin by Fery, but unfortunately using the difficult precursor acetic anhydride. Warfarin is a
coumarin derivative, but it seems better to start by directly synthesising 4- hydroxycoumarin, which is a more practical precursor. Warfarin can be
produced in a one-step reaction from that:

(Kleeman-Engels "Pharmaceutical Substances", 4th ed)
And then this thread, where ClearlyNotAtara outlines a synth from Aspirin and malonic acid, casually passing 4-hydroxycoumarin on the way:
(https://www.sciencemadness.org/whisper/viewthread.php?tid=16...)
| Quote: |
acetylsalicylic acid + NaOEt + EtOH + ∆ >> sodium acetylsalicylate + EtOH (g) i.e. just make the sodium salt alkoxide is used here or use
something else
sodium acetylsalicylate + EtBr >> NaBr + ethyl acetylsalicylate
ethyl acetylsalicylate + NaOEt (dry) >> 4-hydroxycoumarin (2,4-dioxodihydrobenzopyran) [1] |
To begin from something as accessible as acetylsalicylic acid is a very tempting approach, but I must admit I don't quite follow the reasoning behind
the route, especially not after reviewing the sources:
[1] http://www.orgsyn.org/demo.aspx?prep=cv1p0235
[2]http://orgsyn.org/demo.aspx?prep=CV1P0149
[3]https://sci-hub.st/10.1016/j.saa.2006.08.023
And that, I'm afraid, is all I'm able to find on this site.
Moving outside, I had little luck with OrgSyn both for warfarin and 4-hydroxycoumarin.
Did find a source for a route from phenol (https://www.sciencedirect.com/science/article/pii/S187853521...):
| Quote: |
5.2.2. Using phenol
Heating of phenol with malonic acid in phosphorus oxychloride containing twofold amount of anhydrous zinc chloride yielded 4-hydroxycoumarin 1 (Naveen
et al., 2006) (Scheme 4).
|
but it kind of falls flat due to the use of something as inaccessible as phosphorus oxychloride. Several syntheses were also excluded for being too
complicated, using really exotic precursors, or just reagents that are illegal or hard to come by for the home scientist. In this group I
unfortunately must include acetic anhydride, since it is on the top-tier list of narcotics precursors in all of EU. No simple rat murderer wants to be
suspected of cooking heroin. That rules out elegant solutions like this:
https://www.youtube.com/watch?v=oDakl9zxwE4
Youtube is littered with Indian guys scribbling more or less the same synthesis on whiteboards and notebook pages.
Materials and methods
Since I still haven't been able to find a feasible route for this synth using realistic reagents, I need community help. The search ability of people
on this site always amazes me.
I've yet to find a path from the rather easily made hymecromone (7-hydroxy-4-methylcoumarin: https://www.youtube.com/watch?v=1OmL1odquqo to anything useful, for example.
Also, I need help thinking outside my warfarin box. Is there another good rat poison that is easier to cook up? Any suggestions are much appreciated.
[Edited on 20232323/4/2 by DocX]
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by DocX  | And then this thread, where ClearlyNotAtara outlines a synth from Aspirin and malonic acid, casually passing 4-hydroxycoumarin on the way:
| Quote: |
acetylsalicylic acid + NaOEt + EtOH + ∆ >> sodium acetylsalicylate + EtOH (g) i.e. just make the sodium salt alkoxide is used here or use
something else
sodium acetylsalicylate + EtBr >> NaBr + ethyl acetylsalicylate
ethyl acetylsalicylate + NaOEt (dry) >> 4-hydroxycoumarin (2,4-dioxodihydrobenzopyran) [1] |
|
Could you please post the link to this?
|
|
|
SplendidAcylation
Hazard to Others
  
Posts: 196
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
Quote: Originally posted by DocX  | Background
But the internal bleedings caused by warfarin will not be fatal until a day or two later.
It's an attractive solution.
|
Jesus.
Poor things, I think I'll sit this one out...

|
|
|
Lionel Spanner
Hazard to Others
  
Posts: 163
Registered: 14-12-2021
Location: near Barnsley, UK
Member Is Offline
|
|
The Pechmann synthesis for coumarins uses conc. sulphuric acid and aluminium chloride, rather than phosphoryl chloride - using malonic acid as the
beta-carbonyl compound should in theory give you 4-hydroxycoumarin.
|
|
|
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
Yes, sorry, it was meant to have been linked in the word "this". I added it as a clear source link.
|
|
|
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
I think it's probably the kindest rat-fighting method there is. "Internal bleedings" has a dramatic ring to it, but they're actually not painful and
usually occur in the gastrointestinal tract, in rats as in humans. You die from painless blood loss, which means you get drowsy and then loose
consciousness. Sure beats rat traps.
As for killing rats in any way: the fight between us and them has been ongoing since ... I guess since for as long as we both have been around? Funny
thing is, the amount of rats in an area increases almost linearly with the amount of humans. From what I've heard, they're almost always twice as many
as we are. So in a way, they're like livestock or pets: only exists because we do. Too bad they show their gratitude by munching away on the wires in
the main fuse board ...
I just want an acre where my kids can sleep safely without the risk of getting burnt alive. Internal bleedings or not.
[Edited on 20232323/4/2 by DocX]
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Thanks. It looks like what atara proposed is a clever intramolecular Claisen condensation of ethyl salicylate using sodium ethoxide. Like so:
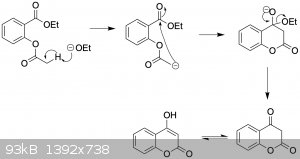
I did a Sci-Finder search and it has been done before, but only in a Chinese patent with 20% yield, using the methyl analog rather than ethyl.
https://worldwide.espacenet.com/patent/search/family/0050314...
The more popular routes all appear to go via 2'-hydroxyacetophenone, for which there are numerous methods.
(This is what me procrastinating on an important presentation for school looks like)
|
|
|
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Lionel Spanner  | | The Pechmann synthesis for coumarins uses conc. sulphuric acid and aluminium chloride, rather than phosphoryl chloride - using malonic acid as the
beta-carbonyl compound should in theory give you 4-hydroxycoumarin. |
Yes! Thank you! I instantly latch onto that.
Phenol + malonic acid in a solution of aluminium chloride and ... yes, what solvent should there be? Should there be heat? How much? For how long? Oh
those devilish details.
I find this reference: http://www.chm.bris.ac.uk/motm/warfarin/preparation.html, which doesn't offer much in terms of details.
But then there's this one: https://pubs.acs.org/doi/pdf/10.1021/acsomega.9b00257
giving us gems like this:

So, according to that; no solvent, 3 h at 130 C, reported yield around 80-90%.
And from what I gather, an 1:1:1 molar ratio of reagents. Or wait, am I reading this right? That's not AlCl3 they have there, it's some exotic
catalyst in a 5 molar ratio... please help.
And then there's this source: https://www.jpsr.pharmainfo.in/Documents/Volumes/vol11issue0...
giving us this reaction:

But that's not 4-hydroxycoumarin as end product now is it? It's some methylated version. And that's not malonic acid as a precursor either, I don't
even know what that is... what even is that?
Is H2SO4 as a catalyst still applicable if phenol + malonic acid is used, and will the reaction then produce the 4-hydroxy version?
If "yes", that would mean we could just mix phenol, malonic acid and conc. sulphuric acid in a beaker, let it sit in room temperature overnight and
then, just maybe, retrieve an 85% yield of 4-OH-coumarin in the morning, as per this figure from the same source:

And then proceed to isolate that from the surrounding phenolic, acidic goo by some steps unknown to me ... God, I really need an experimental to write
one. But if we do manage to isolate it, we can react it with benzal acetone in pyridine to yield ... rat poison!
Not so many OTC reagents there tho.
Phenol I'm sure can be found on Ebay (I know this because I did so)
Pyridine can be made reasonably easy by destructive decarboxylation of niacin (same there).
Malonic acid ... Ebay, apparently.
Aluminium chloride ... nah. But I have to look into the displacement reaction between CuCl2 and aluminium powder. I have some of
both, and they're not difficult to get. Or just react some foil with HCl. Or chlorine gas. But should it be anhydrous or hydrated?
But then again... there's this:
https://eng.hekserij.nl/shop/fragrance-raw-material/search-b...
I hate it when that happens.
Still, there's one step left, and we still need to have some
Benzal acetone, and that's not Hardware Store stock exactly. But acetone is. And sodium hydroxide. And benzaldehyde is achievable if
you google really really hard ... and then there's this: http://orgsyn.org/demo.aspx?prep=CV1P0077.
It's starting to take form. But for A Bear Of Little Brain like me, much reading still needs to be done.
[Edited on 20232323/4/3 by DocX]
[Edited on 20232323/4/3 by DocX]
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
I've had to disconnect SEVERAL circuits running to the far end of my house due to the damn rats chewing on the wires in the attic. To get power to
that part of the house I now have a piece of romex running down my hall up against the ceiling where I can SEE it and the rats can't chew at their
leisure.
Good Luck in the age old battle DocX!
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Warfarin is slow. It lasts days until the coagulation protein level falls down. It blocks synthesis of one of these proteins in liver. Half time of
this protein is something like 1 week. Antidotum is vitamin K (eat a lot of tomatoes, cabbage, lettuce etc when working with warfarin). In case of
severe bleeding there is no time to wait for synthesis of these proteins and human plasma is administered to the patient (which includes this ready
protein from donor people). The usual doses of warfarin administered to people are something like 2-5 mg per day. This dose is therapeutic, not
lethal.
Rats are allegedly clever. When finding new source of food, only very few of them feed on this newly found food, the rest of the group waits and start
to feed on new food only after some time passes. Here the advantage of warfarin. If using fast poison like metal phosphides (which hydrolyze with HCl
in stomach to gaseous PH3) the rest of the group may see the new food is not good to eat.
There could be also predators feeding on rats, if any predator eats warfarin poisoned rat the predator could also die.
[Edited on 3-4-2023 by Fery]
|
|
|
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Fery  | Warfarin is slow. It lasts days until the coagulation protein level falls down. It blocks synthesis of one of these proteins in liver. Half time of
this protein is something like 1 week. Antidotum is vitamin K (eat a lot of tomatoes, cabbage, lettuce etc when working with warfarin). In case of
severe bleeding there is no time to wait for synthesis of these proteins and human plasma is administered to the patient (which includes this ready
protein from donor people). The usual doses of warfarin administered to people are something like 2-5 mg per day. This dose is therapeutic, not
lethal.
Rats are allegedly clever. When finding new source of food, only very few of them feed on this newly found food, the rest of the group waits and start
to feed on new food only after some time passes. Here the advantage of warfarin. If using fast poison like metal phosphides (which hydrolyze with HCl
in stomach to gaseous PH3) the rest of the group may see the new food is not good to eat.
There could be also predators feeding on rats, if any predator eats warfarin poisoned rat the predator could also die.
[Edited on 3-4-2023 by Fery] |
Not THAT slow. When you put a patient on warfarin, you use the doses you say, and then it takes 1-2 weeks to reach stable plasma concentrations of
therapeutic levels. But, also like you say, those doses are tiny- because the therapeutic range of warfarin is really narrow. You measure it by
secondary endpoints- one part of the wonderfully intricate blood clotting cascade of co-dependent enzymatic events, the prothrombin complex.
But that measurement can actually shift daily, and there was, and is, a costly struggle to keep up with changing old kidneys and livers of patients
taking the medication. That's why health care personnel got so used to dealing with warfarin intoxications: a dehydrated old lady who had a fall,
possibly a minor head trauma, and was found to have blood clotting levels through the roof - it was an almost daily occurrence at the ER. Dangerous,
very costly events.
That's why warfarin for the most part has been phased out in favour of the so called NOAC (new oral anticoagulants) medicines, which have much more
stable pharmacodynamics and wider therapeutic ranges.
But the rats are given HUMONGOUS doses compared to. little old ladies. it shouldn't take them more than a day or so to die.
But how about the chemistry? If one should go with Pechmann, would H2SO4 be feasible?
|
|
|
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
Actually... reviewing my own digging, I see an error in my thinking.
If I was to skip the synthesis of 4-hydroxycoumarin from phenol and instead buy the easily available Coumarin - is there actually an easy way to
convert that to 4-hydroxycoumarin?
So the route would be
COUMARIN --[unknown reaction]--> 4-HYDROXYCOUMARIN --[benzal acetone, pyridine]--> WARFARIN
[Edited on 20232323/4/3 by DocX]
[Edited on 20232323/4/3 by DocX]
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Unfortunately I think you're actually taking a step in the wrong direction. I can't think of any conditions that would affect that oxidation
selectively, and SciFinder doesn't bring anything up.
|
|
|
Lionel Spanner
Hazard to Others
  
Posts: 163
Registered: 14-12-2021
Location: near Barnsley, UK
Member Is Offline
|
|
Found this 1943 paper, which details the preparation of 4-hydroxycoumarins from methyl acetylsalicylate (i.e. the methyl ester of aspirin.)
https://pubs.acs.org/doi/pdf/10.1021/ja01252a007
|
|
|
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
Amazing find! Alas, no access. I'll have to fix that.
[Edited on 20232323/4/4 by DocX]
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
https://sci-hub.se/https://doi.org/10.1021/ja01252a007
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DocX  | Background
It now costs well over €1000 for a first visit, and if you want the pest control to continue, you're forced into buying a subscription to their
services ... you get the idea. Net cost can land you well above €2000.
This calls for rebellion.
[Edited on 20232323/4/2 by DocX] |
...Or a cat.
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Quote: Originally posted by Texium  | | I did a Sci-Finder search and it has been done before, but only in a Chinese patent with 20% yield, using the methyl analog rather than ethyl.
|
Done in a German paper from 1915 from methyl acetylsalicylicate in 55% yield: H. Pauly, K. Lockemann, Berichte der deutschen chemischen Gesellschaft,
48, 1, p. 31, https://doi.org/10.1002/cber.19150480107
I'll translate the relevant section here:
| Quote: |
100 g of pure acetylsalicylic acid methyl ester are heated in an open flask in an oil bath to 165 °C. 12 g of sodium metal is added in thin slices,
at a rate similar to their dissolution. The pieces should be submerged immediately and distributed quickly, and the temperature should not rise above
175, but also not drop below 160 °C. The precipitation of a brown colored sodium compound begins instantly, and at the end the whole mass is solid
and dry. At this moment, adding the last bits of sodium are prone to overheating and finally charring, but this can be easily prevented by cooling the
flask in cold paraffin. The hard viscous mass is ground under high boiling petrol ether and after its removal is added to mineral acid, where the
formed benzotetronic acid [oxycoumarin] soon precipitates in plate like crystals. After recrystallization from 70% ethanol the melting point was
correctly 206 °C and free of salicylic acid. Obtained 43-44 g. |
we apologize for the inconvenience
|
|
|
Texium
Administrator
       
Posts: 4508
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Sheesh, not sure how I managed to miss those papers. I had done a structure search and normally that will pull up everything, regardless of language.
Edit: probably because I was searching for routes using alkoxides rather than sodium metal. I had seen some routes that used sodium metal, but had
assumed that wasn’t accessible enough for OP’s needs.
[Edited on 4-5-2023 by Texium]
|
|
|
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
Great idea! What is the best route from a base polycarbonate to a full cat? Can't find it on SciFinder.
|
|
|
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Diachrynic  | Quote: Originally posted by Texium  | | I did a Sci-Finder search and it has been done before, but only in a Chinese patent with 20% yield, using the methyl analog rather than ethyl.
|
Done in a German paper from 1915 from methyl acetylsalicylicate in 55% yield: H. Pauly, K. Lockemann, Berichte der deutschen chemischen Gesellschaft,
48, 1, p. 31, https://doi.org/10.1002/cber.19150480107
I'll translate the relevant section here:
| Quote: |
100 g of pure acetylsalicylic acid methyl ester are heated in an open flask in an oil bath to 165 °C. 12 g of sodium metal is added in thin slices,
at a rate similar to their dissolution. The pieces should be submerged immediately and distributed quickly, and the temperature should not rise above
175, but also not drop below 160 °C. The precipitation of a brown colored sodium compound begins instantly, and at the end the whole mass is solid
and dry. At this moment, adding the last bits of sodium are prone to overheating and finally charring, but this can be easily prevented by cooling the
flask in cold paraffin. The hard viscous mass is ground under high boiling petrol ether and after its removal is added to mineral acid, where the
formed benzotetronic acid [oxycoumarin] soon precipitates in plate like crystals. After recrystallization from 70% ethanol the melting point was
correctly 206 °C and free of salicylic acid. Obtained 43-44 g. |
|
This seems seductively doable. But also prone to errors. Charring? Those dry synths at high temps sort of scare me. I see pictures of charred and
destroyed RBF:s flash before my eyes.
Also, this version is actually tried and mentioned in the paper referred to by Lionel Spanner above, and found wanting:
| Quote: |
Pauly and Lockemann" synthesized 4-hydroxy- coumarin from methyl acetylsalicylate by adding metallic sodium to the molten ester. They re- ported a
yield of 55%. However, by following their conditions we were not able, in spite of many trials, to obtain pure 4-hydroxycoumarin in yields above 13%.
|
[Edited on 20232323/4/5 by DocX]
[Edited on 20232323/4/5 by DocX]
|
|
|
Diachrynic
Hazard to Others
  
Posts: 219
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
Quote: Originally posted by DocX  | | Also, this version is actually tried and mentioned in the paper referred to by Lionel Spanner above, and found wanting |
That's a shame. Thanks, that's important information.
we apologize for the inconvenience
|
|
|
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Unfortunately I think you're actually taking a step in the wrong direction. I can't think of any conditions that would affect that oxidation
selectively, and SciFinder doesn't bring anything up. |
Yeah, I can see that. Except for biosynthesis that is, but those are messy.
My initial hunch to go for the 4-hydroxy version directly is probably the way to go.
I had an answer written to your post above about 2'-hydroxyacetophenone- routes too, but for some reason I get an error whenever I try to post it. At
least one of those routes seems perfectly doable and well describe, but I can't find an accessible source of the starting material.
Whenever I try to answer that post with a link to sci-hub about a specific synthesis I get the error message:
| Quote: |
The system has failed to process your request. If you're an administrator, please set the DEBUG flag to true in config.php.
|
[Edited on 20232323/4/7 by DocX]
|
|
|
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
Trying again (the thing that brought on the error seems to have been a character in the quote, a degree symbol after 100 in the quoted text from the
paper):
Quote: Originally posted by Texium  |
The more popular routes all appear to go via 2'-hydroxyacetophenone, for which there are numerous methods.
|
Ok! New to me. Found this:
https://sci-hub.st/10.1081/scc-100104003
| Quote: |
General Procedure for the Preparation of 4-Hydroxycoumarin (3a)
To a stirred suspension at room temperature sodium hydride (60% in mineral oil, 60mmol) in anhydrous toluene (30mL) was added dropwise a solution of
2-hydroxyacetophenone (12mmol) and acylating reagent (18mmol) in anhydrous toluene (6mL). The reaction mixture was heated at 100C for 3h. After
cooling, the solvent was evaporated and the residue was treated with water (10 mL). The resulting solid was filtered, washed with water, and then
recrystallized from methanol or ethanol to give 4-hydroxy- coumarin as a pale yellow crystal. |
But how oh how does one get access to dat 2'-hydroxyacetophenone?
[Edited on 20232323/4/7 by DocX]
[Edited on 20232323/4/7 by DocX]
|
|
|
Mateo_swe
National Hazard
   
Posts: 505
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
Poor rats, they are living animals too.
If you need to kill them, do it quickly.
Just put a big tub filled with water then a thin round pole smeared with lubricant over its opening at the top.
Put a piece of food at the center of the pole.
The rat or mice will go out on the pole and loose grip falling inte the water and drown.
Or if you use a tub with high walls you can catch them alive and drive them out in the forrest somewhere, thats seems like the friendliest thing.
Better than rats to go around like zombies for days or even weeks, probably not plesant.
I have seen what Warfarin does to rats, it doesnt seem nice at all, there must be better ways.
A cruching trap would be best but if you have a big rat infestation thats going to be hard to get them all with such trap.
Regarding the chemistry, intresting..
|
|
|
| Pages:
1
2 |