AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
vicinal amino-nitroalkanes ?
Are there any instances of a stable compound with a nitro group on a carbon atom adjacent to another carbon with an amino group, in which the two
carbon atoms are single bonded? For example, 2-nitroethylamine with the structure
NH2CH2CH2NO2
Obviously 1-nitro-2-amino aromatic rings rings exist, but this is not what I am asking about.
I have a suspicion that 1-nitro-2-amines of alkanes are inherently unstable. There do not seem to be any solid references about such compounds.
A search did not reveal much:
"Heath and Rose reported the synthesis of crude 2-nitroethylamine, and noted that it decomposed to a black tar in 1–2 hours. It is uncertain whether
this..."
http://www.sciencedirect.com/science?_ob=ArticleURL&_udi...
Another article describes chemically reducing "thermally unstable 2-aminonitroalkanes".
"Reactions between nitrogen nucleophiles and nitroalkenes provide a rapid route to 2-aminonitroalkanes, which, after reduction of of the nitro group,
supply vicinal diamino derivitives. Michael addition of O-ethylhydroxylamine to nitroalkenes produces the corresponding 2-hydroxylamino
nitroalkanes.”
Amino group chemistry: from synthesis to the life sciences. Alfredo Ricci, p106-107
Here is an ambiguous diagram, not sure whether it is saying the nitro-amine decomposes, or whether it is being reacted with something else to yield
the indicated products:
https://www.thieme-connect.com/media/synthesis/201018/p063_s...
While thinking about the reaction of nitromethane with bases, I was wondering why the 2-nitroethanaloximate which forms does not condense further with
another molecule of nitromethane to give a compound with the structure O2NCH2CH(NHOH)CH2NO2. I think it is possible such a condensation actually takes
place, but that the resulting product can revert back, in a similar way perhaps to decomposition of 1-nitro-2-amino alkanes.
I am not sure exactly what the reaction mechanism would be for the degradation of nitro-amino alkanes, perhaps something like what is indicated in the
diagram I made below:
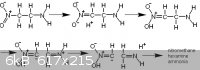
Alternatively, the amine could simply donate an electron towards the opposite carbon atom, the electron then quickly being withdrawn into the nitro
group.
NH2CH2CH2NO2 --> NH2(+)=CH2 (-)CH2NO2 --> (+)H NH=CH2 CH2=NO2(-)
[Edited on 22-4-2011 by AndersHoveland]
|
|
|
Ebao-lu
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
b-aminonitroalkanes decompose due to b-elimination (E1cb elimination) with formation of nitroalkenes and amine. Nitroalkene can then undergo Michael
addition to other amino-groups or to enolized nitroalkane groups and polymerize(with amine catalysis)
If aminogroup is in gamma, delta etc positions , then such aminonitroalkanes are stable. Also, if you can substitute all hydrogens in a-position with
methyl, aminomethyl, hydroxymethyl etc groups - then b-aminonitroalkanes would also become stable.
| Quote: | | I was wondering why the 2-nitroethanaloximate which forms does not condense further with another molecule of nitromethane to give a compound with the
structure O2NCH2CH(NHOH)CH2NO2. |
that is i think because 2-nitroethanaldoxime is present in one of its enolized forms (CH=N(O)OH or CH=CH-NHOH), both are highly stabilized by
conjugation, delocalization of charge, intermolecular H bond (that also makes them convert into each other). When nitromethane's aci-form attacks at
b-position, you lose all this conjugation and stabilization, so this is not beneficial (or as you said, " it is possible such a condensation actually
takes place, but that the resulting product can revert back"). Besides, is it is present as aldoximATE, as you said (due to this anion is highly
stabilized), you lose any ability of this molecule to react with nuckleophiles because there is not sufficient EWG stabilization for anion, that is
formed after nucleophilic attack
| Quote: | | Alternatively, the amine could simply donate an electron towards the opposite carbon atom, the electron then quickly being withdrawn into the nitro
group. |
amine can't donate electrons if there is no adjacent double bond it is conjugated with
[Edited on 4-5-2011 by Ebao-lu]
[Edited on 4-5-2011 by Ebao-lu]
[Edited on 4-5-2011 by Ebao-lu]
[Edited on 4-5-2011 by Ebao-lu]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Interesting theory you have about the resonance structures/ aromaticity of the 2-nitroethanaloximate ion.
Presumably this is what you mean:
(-)O2N=CHCH=NOH <--> O2NC(-)CH=NOH <--> O2NC=CHN(-)OH
If this is the case, it likely means that the 2-nitroethanaloximate ion does not exist as a trimer.
quote: "amine can't donate electrons if there is no adjacent double bond it is conjugated with"
I understand what you are saying and that is generally true when discussing aromaticity within a molecule, but nevertheless amines can donate
electrons to electron-withdrawing groups, which subsequently break off the molecule. NH2CH2Cl for example does not exist, as the chlorine atom would
ionize off.
quote: "if you can substitute all hydrogens in a-position with methyl, aminomethyl, hydroxymethyl etc groups - then b-aminonitroalkanes would also
become stable"
This would only be true if the only decomposition route of 1-nitro-2-aminoethane was the ionization of the amine off the molecule leaving
nitroethylene. From one of the pictures in the links I posted, it would appear this may be only one of two possible decomposition routes, meaning
addition of methyl groups would not provide stability.
https://www.thieme-connect.com/media/synthesis/201018/p063_s...
The other pathway seems like the one I originally proposed, although not sure what the "PG" group means.
[Edited on 4-5-2011 by AndersHoveland]
|
|
|
Ebao-lu
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
| Quote: | | If this is the case, it likely means that the 2-nitroethanaloximate ion does not exist as a trimer. |
it will
hardly exist as trimer also because of electrostatic repulsion, i suppose
Yes, that was what i meant, and aromaticity can result from hydrogen bonding : the left structure that wou depicted forms intramolecular hydrogen bond
(NOO-....H-O-N= ), and it also can become (NO-OH....-O-N=) so hydrogen is like a bridge making a cycle, that results in another charge delocalization
possibilities:
(OH)2N(-)CH=CH-N=O <--> HO2N=C-CH(-)N=O <--> HO2N=C-CH=NO(-)
not sure about aromaticity and proton bridge formation yet, but it seems possible for me
[edited: no, obviosly it is not aromatic. tried to find something about the anion structure (there is also dianion btw), so maybe a proposal about
hydrogen bridge was also not correct]
[Edited on 4-5-2011 by Ebao-lu]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Yes, this seems like it could be quite possible... very interesting. Such a salt is very deserving of detailed professional study.
|
|
|
Ebao-lu
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
| Quote: | From one of the pictures in the links I posted, it would appear this may be only one of two possible decomposition routes, meaning addition of methyl
groups would not provide stability.
https://www.thieme-connect.com/media/synthesis/201018/p063_s...
The other pathway seems like the one I originally proposed, although not sure what the "PG" group means. |
I think, PG is protecting group here. Also, i will not be surprised if it is an EWG group that stabilizes N(-) and allows to abstract hydrogen with
base, and nitroalkane leaves as anion. Otherwise, you need to cleave b-aminonitroalkane into imminium cation and nitroalkane anion(heterolytically),
that requires much energy and will make activation energy higher. At the same time, if PG is EWG group, it seems that HN(-)PG is worse then NH3 as
leaving group, because CH2-NHPG can not become protonated, while CH2-NH2 can. That means that protection of nitrogen with EWG group can save it from
b-elimination, so the main pathway in basic media is cleavage into nitroalkane and PG-imine
[Edited on 4-5-2011 by Ebao-lu]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
"edited: no, obviosly it is not aromatic. tried to find something about the anion structure (there is also dianion btw), so maybe a proposal about
hydrogen bridge was also not correct"
nitroformate (O2N)2C=NO2(-) is an aromatic ion (which is bright yellow colored as a result). During the formation of 2-nitroethanaloximate (from a
base and nitromethane), a dark reddish-brown color slowly develops, although supposedly the pure crystals of 2-nitroethanaloximate salts are
colorless. This would suggest that something in the solution has an aromatic structure, but it could just be entirely attributable to a polymerized
byproduct.
I have not read anything about the double (-2) charged 2-nitroethanaloximate. One would think it may be possible, but the formulas in the literature
did not mention anything about these other salts. It stated that the lead salts are insoluble, which may suggest a (-2) charge, but it did not give
any formula for this. Your idea about possible interpolyatomic ion aromaticity across hydrogen bonds does not seem completely implausible to me. But
of course, we really do not know whether this is the case, or if there is any aromaticity within a single polyatomic ion. It is also possible that
interesting resonance states do not exist at all, in which case the molecule may lhave either of the structures below,
O2NCH2CH=NO(-) , (-)O2N=CHCH=NOH
What do you mean by "EWG" group?
[Edited on 4-5-2011 by AndersHoveland]
|
|
|
Ebao-lu
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
EWG = electron withdrawing group (like COOR, COR, CN, NO2 etc) that can stabilize "-" on adjacent atom ( (-)NR-CN <-> RN=C=N(-) )
Color is not an indication of aromaticity, aromatic pi-system shuld be cyclic and have 4n+2 pi electrons, where n is any integer. In case of H-bridged
cathion we have, if i am not mistaken, 8 electrons - so it is antiaromatic. Not cylclized cathion has 6 electrons, but it is not aromatic because not
cyclic.
As for diainon, search in google for "disodium methazonate". If you have access(i don't) to the papers, maybe you can see the actual resonance
structures for it (like this one http://pubs.acs.org/doi/abs/10.1021/jo01045a533)
[Edited on 5-5-2011 by Ebao-lu]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
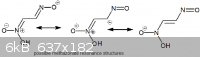
Methazonate Anion Resonance
The first image below shows possible resonance structures for the methazonate anion. This may explain why the oxime does not trimerize, and why the
methazonate does not condense further with another molecule of nitromethane.
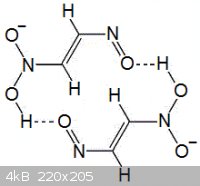
The second image below shows a possible dimer of two methazonate anions, with intermolecular aromaticity across and through hydrogen bonds. Perhaps
this aromatic dimer has some equilibrium in solution, but is not favored by crystal packing in the solid crystalline form. This might explain why the
alkaline nitromethane solutions turn dark red-brown (because of conjugated bonding), yet pure methazonate salts tend to be colorless. For
psuedonitrosite compounds, nitroso groups on two different molecules are already known to dimerize, resulting in a color change.
[Edited on 5-5-2011 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
"Disodium methazonate was prepared by the action of concentrated sodium hydroxide on nitromethane and the free acid and the monosodium salt
isolated..."
Apparently both the monosodium and disodium salts of 2-nitroethanaloxime, in addition to the free acid, have been prepared and isolated. Seems to be
only a matter of pH.
|
|
|
|