Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Menthol
| Quote: |
Menthol
Menthol is an organic compound made synthetically or obtained from peppermint or other mint oils. It is a waxy, crystalline substance, clear or white
in color, which is solid at room temperature and melts slightly above. The main form of menthol occurring in nature is ( − )-menthol, which is
assigned the (1R,2S,5R) configuration. Menthol has local anesthetic and counterirritant qualities, and it is widely used to relieve minor throat
irritation. Menthol also acts as a weak kappa Opioid receptor agonist.
As with many widely-used natural products, the demand for menthol greatly exceeds the supply from natural sources. Menthol is manufactured as a single
enantiomer (94% ee) on the scale of 3,000 tons per year by Takasago International Corporation. The process involves an asymmetric synthesis developed
by a team led by Ryōji Noyori:
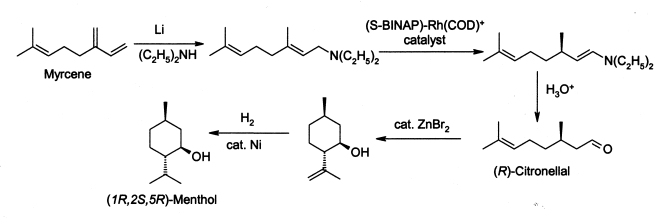
|
| Quote: |
Racemic menthol can be prepared simply by hydrogenation of thymol |
| Quote: |
(-)-Menthol Synthesis from m-Cresol / Thymol
|
Anyone know what pressure and condition is needed for making thymol from m-cresol with propylene?or anyone know better method for making menthol?
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Another interesting method:
| Quote: |
Menthol from Mesityl oxide via Piperitenone
|picture removed|
In early 2002, Takasago was issued a patent for the preparation of (-)-menthol from mesityl oxide via piperitenone. As with their commercial process
via myrcene, this synthesis utilizes chiral hydrogenation catalysts at several stages. The key step is the conversion of piperitenone to (+)-pulegone
in about 90% yield with enantioselectivity in the range of 98%.
While it is not expected that this process will replace the successful myrcene process, it provides Takasago with another option if myrcene is
difficult to obtain.
This patent is available for download below
|
This is possible to hydrogenation Piperitenone to Racemic Menthol?and then resolution Racemic Menthol?
What pressure needed for this reaction?
Attachment: menthol from piperitenone.pdf (1MB)
This file has been downloaded 419 times
Edit by Nicodem: Removed the linked-in picture that Waffles SS plagiarized from another site together with the text
bellow it, because it was meanwhile changed into something improper for this forum.
[Edited on 1/6/2012 by Nicodem]
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
Why the interest Waffles? The only one of the precursors that you have mentioned that is OTC cheaper than menthol is citronellal.
We are at the opposite end of the supply chain from the industrialists. Menthol is readily available by the kilogram. If it could be cheaply turned
into something more unusual, interesting or expensive.... now that would be worthwhile.
Thymol is used by beekeepers to control varroa mites, but it is very expensive ... something like NZ$400 for 500g last time I looked. Can it be made
from menthol?
Of course if there is a demand for menthol the price will inevitably go up.
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
Why not interesting? making citronella is difficult and need certain catalysist(BINAP rhodium complex) but i think Piperitenone method is simpler.Here
menthol is 50-60$/kg
Also i think making menthol from m-Cresol / Thymol isnot very difficult if we know detail in this reaction(pressure,temp).I have access to Propylene
gas , M-Cresol and also i have high Pressure Autoclave(i like to try this method)
Attachment: AECI_menthol.pdf (830kB)
This file has been downloaded 417 times
Attachment: menthol from piperitenone.pdf (1MB)
This file has been downloaded 461 times
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
In Thymol method after hydrogenation we will get a mix of menthol, neomenthol, isomenthol and neoisomenthol - that's 4 diastereomers Each isomer can
either have (+) or (-) configuration - that's 2 enantiomers.
Since we will end up with a racemate of each, we will be dealing with four "pairs of diastereomers" - that's 8 different substances.
Fractional distillation will leave us with a fraction containing the (+)- and the (-)-menthol - a racemate again.
Resolution of the racemate can be carried out by the ways already mentioned .
The rest of the distillates, containing the abovementioned neomenthol, isomenthol and neoisomenthol racemates is epimerized (or "isomerized"), partly
to racemic menthol and fed back into the production process and i want to know how this isomerization/epimerization process works
Sometimes, isomerization/epimerization can be done by heating with a base (e.g., quinoline in the case of gluconic acid), sometimes by heating with an
acid (e.g., p-tosic acid, in the case of a certain ...hmm ... let us call it "mind altering substance"). Sometimes even plain heating in an inert
solvent will work but what about menthol isomers?
"original post by my dear friend:Obtainium in http://www.versuchschemie.de"
http://www.versuchschemie.de/hartmut.php?t=15478&postday...
[Edited on 23-6-2011 by Waffles SS]
|
|
|