IPN
Hazard to Others
  
Posts: 156
Registered: 31-5-2003
Location: Finland
Member Is Offline
Mood: oxidized
|
|
Help with quinoline synthesis
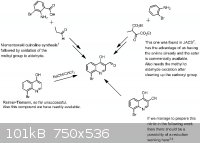
We have been puzzled by a synthesis problem for a while now where our target molecule is a 4-hydroxyquinoline derivative with a formyl group at the
third position.
So far the attempts at the most obvious pathway, a simple Reimer-Tiemann formylation, have been unsuccessful with no detectable product. A paper in JOC reported a 16% yeild for a similar compound but we have been unable to replicate the results for our compound.
Now I'm not looking for the work to be done for me but I'm just interested to know if anyone has some other synthesis ideas on this molecule that we
could explore. 
I have attached a picture which should give a better idea on what we are aiming at with some proposed pathways.
Refs for the pic:
1. Chem. Rev., 1942, 30 (1), pp 113–144
2. J. Am. Chem. Soc., 1946, 68 (7), pp 1232–1238
3. Tet. Lett., 2002, Vol. 43, Is. 8, p. 1395-1396
4. Bull. Korean Chem. Soc. 2006, Vol. 27, No. 1, p. 121 - 122
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
May I suggest using the Duff reaction, see page 66 of this thesis;
http://etheses.nottingham.ac.uk/319/1/final_thesis_as_Pdf.pd...
[Edited on 2-6-2011 by ScienceSquirrel]
|
|
|
IPN
Hazard to Others
  
Posts: 156
Registered: 31-5-2003
Location: Finland
Member Is Offline
Mood: oxidized
|
|
Thank you ScienceSquirrel for the reference! The Duff reaction was tried out, but although it did give the desired aldehyde it did so in only 15%
yield.  Now I don't know if it could be improved, but so far the message I got from
the lab supervisor was that I should look for other routes as TFA is notoriously expensive. Now I don't know if it could be improved, but so far the message I got from
the lab supervisor was that I should look for other routes as TFA is notoriously expensive.
Other things I tried out so far were the synthesis of the 3-methyl substituted 8-bromo-4-hydroxyquinoline through the Conrad-Limpach reaction. This
was found problematic as the imine was a bit hard to make. First the ester and aniline was combined with few drops of conc. H2SO4, put in vacuo over
P2O5 overnight, resulting mixture added to Ph2O, refluxed for 4h, yield 12%. Other attempt at the pure imine formation was by aniline + ester in
toluene, reflux with dean-stark overnight gave 32% pure imine after flash. This in refluxing Ph2O gave 45% yield of the 3-methyl compound based on the
used imine. A synth.comm. article mentioned alternative solvents for the C-L reaction, but at least the 2,6-di-tert-butylphenol they dubbed as "the best solvent" wasn't
working for our substrate as even a 12h reflux didn't yield any precipitate after cooling down. Addition of equal volume of diethylether did
precipitate something crystalline but it did so with the formation of a _lot_ of tar and workup was found impossible (or I just lacked patience.. the
tar clogged frits immediately). I'll try out a way to make the imine similar to the Gould-Jacobs reaction (aniline and ester heater together neat or
maybe with a hint if acid catalyst) and then heat it in Ph2O as at least I know it's relatively insoluble in that.
The 3-methyl substituted product that I managed to isolate was then subjected to SeO2 oxidation (if anyone has anything better in mind for selective
methyl group oxidation to the aldehyde I'd love to know as SeO2 is another expensive and rather toxic reagent) in refluxing xylene. This gave a yield
of 25% which although better than anything previously tried, still sucks. 
The third pathway is looking like the best option so far. This is the synthesis of the 3-substituted nitrile by the Gould-Jacobs reaction from the
aniline and a ethyl (ethoxymethylene)cyanoacetate (both of which are reasonably priced too!  ), and the subsequent reduction of this nitrile to the aldehyde. Preparation of the nitrile was easy (first try at 5 mmol scale yielded 34%
of the nitrile, pure by NMR straight after drying), although it's amazingly insoluble in everything. I can only hope it doesn't matter in the DIBALH
reduction. Other options for the reduction are available but the reagents are more exotic and expensive (LDBBA, PtO2).. ), and the subsequent reduction of this nitrile to the aldehyde. Preparation of the nitrile was easy (first try at 5 mmol scale yielded 34%
of the nitrile, pure by NMR straight after drying), although it's amazingly insoluble in everything. I can only hope it doesn't matter in the DIBALH
reduction. Other options for the reduction are available but the reagents are more exotic and expensive (LDBBA, PtO2)..
That's it for now, hopefully someone will find this interesting. =D
Links for the mentioned reactions if someone isn't familiar with them:
http://en.wikipedia.org/wiki/Gould-Jacobs_reaction
http://en.wikipedia.org/wiki/Conrad-Limpach_synthesis
|
|
|
fledarmus
Hazard to Others
  
Posts: 187
Registered: 23-6-2011
Member Is Offline
Mood: No Mood
|
|
There is a 2006 Synlett synthesis (with no bromo substituent) starting from the aminoacetophenone that might work if you can make the appropriate
brominated starting material:
Coelho et al, Synlett 19 (2006), 3324-3328
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
If you feel inovative, you can try a Mannich reaction with morpholine followed by the oxidation of the Mannich base hydrochloride with DMSO.
The Mannich on your 8-bromo-4-hydroxyquinoline should be high yielding. On plain 4-hydroxyquinoline the yield using morpholine is reported to be 95%
[1]. Similarly on 4-hydroxy-2-methylquinoline with diethylamine [2]. There must be plenty other examples in the literature, but I have not bothered
much with the search.
Make sure you use morpholine or some other amine having poorly electrophilic methylene groups in the Mannich base (don't use dimethylamine!).
The Kornblum on benzylammonium salts has been reported [3]. Since amines are very poor leaving groups, the reaction requires harsh conditions (up to
180 °C). The Mannich base has a hydroxy group ortho to the aminomethylene group, so that it is prone to elimination forming the corresponding
ortho-quinomethane. These are highly electrophilic and should easily O-alkylate DMSO and do the Kornblum thing. This might allow the reaction to proceed
at lower temperatures.
I would also not be surprised at all if a one-pot reaction starting from the 8-bromo-4-hydroxyquinoline, trioxane and morpholine hydrochloride in DMSO
turns out feasible.
On the other hand, heating the Mannich base in DMSO might only give crap. But you can't be sure until you try. If for some strange coincidence it
turns out a success, make sure you do not forget citing Sciencemadness in your report/article. Don't make me writing to the editors! 
[1] Bulletin of the Korean Chemical Society 1999, 20, 973-976. (freely availble online)
[2] Canadian Journal of Chemistry 1966, 44, 1863-1865. (old issues are freely available, I think)
[3] J. Org. Chem. 1970, 35, 2207–2212.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
IPN
Hazard to Others
  
Posts: 156
Registered: 31-5-2003
Location: Finland
Member Is Offline
Mood: oxidized
|
|
Thank you fledarmus! The article isn't available to me online, but I'll be sure to check it on monday when I visit the library. 
Very interesting stuff on the Mannich Nicodem, thanks a lot!  I was going to try
the Kornblum on the 3-methyl substituted one (after bromination with NBS), but that is for when all else fails. This would be much better though,
skipping few steps too. Then again it might be too good to be true, but as you said, I need to try it to know (and I'll remember scimad if it does I was going to try
the Kornblum on the 3-methyl substituted one (after bromination with NBS), but that is for when all else fails. This would be much better though,
skipping few steps too. Then again it might be too good to be true, but as you said, I need to try it to know (and I'll remember scimad if it does
 )! And all that Me2S aroma will mix nicely with the mercaptan stuff going on in
the fumehood next to me. )! And all that Me2S aroma will mix nicely with the mercaptan stuff going on in
the fumehood next to me. 
|
|
|
IPN
Hazard to Others
  
Posts: 156
Registered: 31-5-2003
Location: Finland
Member Is Offline
Mood: oxidized
|
|
So the DMSO oxidation was tried out but unfortunately no aldehyde was found in the extracts after workup.  I used some previously prepared mannich bases, made with piperidine and morpholine. These were around 2 months old,
but were still pure by NMR so I doubt the fault was in the starting materials. Hydrochlorides were prepared by gassing with HCl in DCM
suspension/solution. I used some previously prepared mannich bases, made with piperidine and morpholine. These were around 2 months old,
but were still pure by NMR so I doubt the fault was in the starting materials. Hydrochlorides were prepared by gassing with HCl in DCM
suspension/solution.
On 1mmol scale the DMSO amount used in the JOC article wasn't enough to result in a stirrable mixture so I don't know if this contributed to the
black, tarry reaction mixtures after the 20h heating period (under argon). Smell of dimethyl sulfide was very strong (and I had to clean the whole
schlenk line because I used the argon line and not a balloon, silly me) but this was to be expected. Workup was done with 1M HCl / brine / EtOAc. It
might be that an inert, high-boiling solvent could help in preventing over oxidation/tar formation but unfortunately I don't have enough time to
explore this pathway as much as I'd like.
Nitrile still refuses to get reduced even with fresh DIBAl (I even tried refluxing in one attempt for ~30min, no reduction  ). The 4-OH-quinoline apparently does a good job at complexing with the aluminium
hydrides rendering the reagent inert. ). The 4-OH-quinoline apparently does a good job at complexing with the aluminium
hydrides rendering the reagent inert. 
Oh well, time to start working through a whole different approach..
Anyways, thanks for all the suggestions! 
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Don't be too disappointed, it was a far stretched suggestion anyway. I would be pleasantly surprised if it was successful. I don't have any instantly
useful ideas, but you can try the formylation with HCHO/Mg(OMe)2. See Klute's prepublication for an example and references. There are a couple of other papers with related methods in the literature which are not
listed in that thread, but I'm sure you will be able to find them. I find it unlikely this would work due to the electron poor nature of your
pseudophenol, but if you are desperate you can try it out.
| Quote: | Nitrile still refuses to get reduced even with fresh DIBAl (I even tried refluxing in one attempt for ~30min, no reduction  ). The 4-OH-quinoline apparently does a good job at complexing with the aluminium
hydrides rendering the reagent inert. ). The 4-OH-quinoline apparently does a good job at complexing with the aluminium
hydrides rendering the reagent inert. |
A stupid question, but you did use at least two equivalents of DIBAL-H to compensate for the hydroxy group?
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Also, while you have the nitrile, you can try reducing it with the SnCl2/HCl method.
Raney Ni in formic acid can give acceptable yields of aldehydes from nitriles. US20080132538 has an example of such a method, but there are many other
in the literature (supposedly even the Ni-Al alloy can be used directly).
You can check DOI: 10.1021/cr60120a002 to get ideas on how to introduce that aldehyde group.
|
|
|
IPN
Hazard to Others
  
Posts: 156
Registered: 31-5-2003
Location: Finland
Member Is Offline
Mood: oxidized
|
|
Yeah I used +2eq DIBAL for the nitrile in both THF and DCM, neither showed any reduction products. The Mg-formylation was also attempted but this only
resulted in some funky polymer (dunno if it was the THF, had some trouble dissolving everything in acetonitrile). 
SnCl2/HCl method was looked into as well and it actually seemed like it would have worked as a yellowish precipitate formed shortly after introducing
the nitrile into the Et2O/SnCl4 mix but only starting material was obtained after workup.
I could give the nickel one a go as I'm going to try PtO2 again under more forcing conditions so I can run them in parallel, but most likely I will
find myself with the amine considering how easily the imine would be reduced.. 
|
|
|
IPN
Hazard to Others
  
Posts: 156
Registered: 31-5-2003
Location: Finland
Member Is Offline
Mood: oxidized
|
|
Well, to my amazement the Raney nickel did the job in the microwave reactor. A 50% yield was obtained from the nitrile with a very simple workup. 
The platinum oxide in return gave nothing with the same conditions.
Thanks again Nicodem. 
Now to do the same with some napthyridines... 
|
|
|