ZHANGNIUBI
Harmless

Posts: 28
Registered: 5-5-2011
Member Is Offline
Mood: No Mood
|
|
Sythesis of TEX(with picture in and details..)
Hello, everybody, I am a high school student from China.I love this place. People enjoy explosive like I do...I wanna first apologize for my poor
English. I hope my words won't confuse you guys....
Ok,,I found that the way to sythnesize TEX in the forum is kind of too old and the field is too low... I am not sure if it's suitable to translate a
Chinese literature into English..But the truth is I successfully synthsized some TEX(maybe 5 more gram) in home in few months ago with a field of
about 40%.
now, here is few pictures and the way i did and some intruduction of TEX.
Being Insensitive and high energy has been the goal of an superior explosive. With the need of explosives that are both high energetic and stable ,
more and more explosives are being tried and synthesized these days,TEX is one of them, it has a typical cage-shaped moleculor, with a perfect density
of 1. 99 g / cm 3,under the standard conditions, its friction sensitivity is only 8%, and 44% of impact sensitivity. its detonation performance is
relatively good: calculated detonation velocity is 8 170 m / s(actually I think Vod of TEX is more than 8600m/s,I have data of actual Vod of TEX,when
the density is 1.68g/cm^3,VOD is 7889, 1.65g/CM3,VOD=7776,you can imagine what will happen when D=1.99..) , detonation pressure is 31.4 GPa. Vacuum
stability experiments show that, TEX has a good thermal stability and performance, similar to many performance of TATB. Another advantage is that TEX
can be used for casting of explosives(like TNT does, I am not sure if all sentences make sense...sorry),
TEX full name: 4,10 - dinitro -2,6,8,12 - tetra-oxa -4,10 - diaza Ring [5.5.0.05,903,11] dodecane. As for its structure, famous explosive, CL- 20
(HNIW) is similar to TEX, and the only difference between them is that four oxygen atoms In TEX replace two (=NNO2)bridges in CL-20. Thus, TEX is less
energy than CL-20 but more insensitive and safe!
Here is the translation from Chinese literature..
1, 4-Diformyl-2, 3, 5, 6-tetrahydroxypiperazine (THDFP) w as synthesized with glyoxal and amides as
primary substance. The cage explosive 4, 10-dinitro-2, 6, 8,12-tetraoxa-4, 10-diazatetracyclo-[ 5. 5. 0. 05, 9 03, 11 ]-
dodecane (TEX) was prepared by using the method that taking THDFP into 98% H2SO 4 and fuming HNO3 at lower
temperature, producing enough the cage compound, then carrying out nitration in higher tempreture. The purity
and overall yield of TEX are 99. 5% and 34. 8% , respectivety. The effect of THDFP dry time, the temperature of
adding material and reaction tempreture on the synthesis of TEX were studied. Results show that THDFP of full
dried for synthesis is favored, should controll the temperature of adding material in the range 45~ 50 ℃ and the reaction tempreture in
the range of 75~80 ℃.
THESE ARE BRIEF
HERE ARE SOME Details
1 cyclization
145 g (1mo l) 40% glyoxal aqueous solution is stirring and at the same time, we add the solution of the saturated NaHCO3 in to make sure that the pH
value reaches 8, then add 90 g of formamide (2mol),40-50 degrees Celsius make sure that the precipitation is no longer increase then you can stop
adding . THEN add 100mL of methanol into reactor, and filter, wash several times with methanol at room temperature, you may get the crude product of
112 g(THDPF). The crude product by adding boiling 0.1mol / L hydrochloric acid (700mL) solution, stirring 5mins and quickly cooled, placed at 0
℃, for 2 h, filter, place in silica gel desiccator at room temperature drying, white powder-like crystals is obtained with mass of 70 . 2 g,
yield 68.1%.Then fully dry, then add the quality of the equivalent of 15% urea THDFP quality(THAT MEANS ,FOR EXAMPLE, 100g THDPF should mix with 15g
urea), and further dried (P2O5 drying for several days.)
2 nitrification
Strong stirring the mixture of :40ml fuming nitric acid and 40mL of concentrated sulfuric acid,, and slowly add 10g THDFP and urea
mixture(8.7gTHDPF,1.3g urea), with water cooling the reacter, the temperature should not exceed 50 ℃. 20 ~ 30min after the water bath heated to
the temperature slowly rose to 65~70 ℃ (it's a exothermic reaction, be strict on temperature control !!!), remove the water bath, the reaction
temperature gradually decrease to about 30 ℃, and then put the reaction mixture into ice water(to make it cool down),then filtered(TEX is a kind
of white precipitate, you can see it in the picture), washed by diluted NaHCO3 solution...Finally, wash TEX with ethanol. And then vacuum drying TEX
at 60 ℃ and you can get dry, clean TEX.
The final yield of my experiment was slightly less than the literature, 40%, i finally got about 5g.TEX.
if any sentence makes no sense, please tell me,,,I need to improve my English......and let you guys not be so confused..
Attachment: THDPF,the intermediate of TEX (66kB)
This file has been downloaded 1033 times
Attachment: TEX in the solution of mixture HNO3,H2SO4 (64kB)
This file has been downloaded 968 times
[Edited on 14-6-2011 by ZHANGNIUBI]
[Edited on 14-6-2011 by ZHANGNIUBI]
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ZHANGNIUBI  | | Hello, everybody, I am a high school student from China.I love this place. People enjoy explosive like I do...I wanna first apologize for my poor
English. I hope my words won't confuse you guys... |
http://www.sciencemadness.org/member_publications/TEX.pdf
http://www.sciencemadness.org/talk/viewthread.php?tid=6000
&c., &c.
|
|
|
QHarryQ
Harmless

Posts: 12
Registered: 28-5-2011
Member Is Offline
Mood: No Mood
|
|
It seems to be a regular way to synthsize TEX ,isn't it? But there is no doute that it's an acheivement to synthsize TEX.Congratulations to
you!
|
|
|
ZHANGNIUBI
Harmless

Posts: 28
Registered: 5-5-2011
Member Is Offline
Mood: No Mood
|
|
Thank you, But I want to especially point out three things.
1, when using mixture of HNO3 and H2SO4, it is necessary to use fuming nitric acid(95%+HNO3).
2, Control of the reaction tempurature must be strict, or we may get a low field.
3, there may be something wrong with the data in that literature..NTO,which I also made home before, seems to be a little bit better in performance
than TEX, I am not sure, it may be a little bit debatable..
that's it , I hope these may help..
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
An idea for a different caged molecule that would likely be more powerful than TEX,
https://sites.google.com/site/ecpreparation/tean
A synthesis should be within reach of many of the members here.
[Edited on 16-6-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Very interesting AndersHoveland.
Maybe it can be accessed via I-CH2-CH=O and NH3...
I-CH2-CH=O + 2NH3 --> HN=CH-CH2-NH2.HI
Then upon basification...some of the caged stucture might form via HN=CH-CH2-NH2 and NH3 loss
I wonder if the nitrogen atom without -NO2:
-could be oxydised by H2O2 to make a N=O (tertiary amine oxyde).
-Or if that atom, that should be more basic than the others because of the viccinity of the CH-CH2-, can be protonated by an oxygen carrier... HNO3,
HClO4, HC(NO2)3, HIO4, HN(NO2)3,...
That would make it more sensitive, but also denser and even more powerfull.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Trying to make 2-iodoacetaldehyde, then react it with NH3, would require too much effort, and is likely too problematic to be worth the trouble.
Although BF3 is a poisonous gas, one generally would want to avoid any additional chemicals with poisonous fumes (iodomethane for example is a potent
alkylating agent).
The nitrgoen atom without a nitro group in the cage could be presumably oxidized with H2O2 to the N-oxide, as I am aware of another procedure that
employs a similar oxidation.
(if you are trying to oxidize pyridine to the N-oxide, however, plain H2O2 will not react unless a special zeolite catalyst, known as TS-1 and not yet
commercially available, is used; co-reaction with acetonitrile also allow allows H2O2 to oxidize pyridine to the N-oxide, although the acetonitrile is
simultaneously degredated to acetamide)
The biphosphate salt of triethanolamine trinitrate has been investigated for medical use in treating heart pain, under the label "Metamine", so salts
certainly do exist. It may be possible that a similar nitrate (or perchlorate) salt could not be prepared from the caged compound, TEAN. The salt
would still be acidic, however, and this could result in hydrolysis/degredation of the nitramines. While RDX does not hydrolyze to any appreciable
extent in slightly acidic aqueous solution, I am unsure about the effects of acidity from a nitrate salt. Trimethylammonium chloride, for example, is
fairly acidic, with a pKa of 9.5
For information about the preparation of triethanolamine trinitrate, see http://www.parazite.fi/roguesci/index.php/t-4865.html
Here is the molecular structure of triethanolamine trinitrate, obviously in salts of the compound, the central nitrogen atom would be protonated.
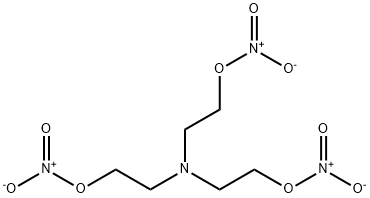
[Edited on 16-6-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I think to remember Triethanolamine makes a tetranitrate compound (TEATeN) via a normal nitration procedure without lipophilic extraction solvent.
N(CH2-CH2-OH)3 + 4HNO3 --> N(CH2-CH2-ONO2)3.HNO3 + 3 H2O
For sure the compound (a salt actually) must be slightly acidic just like NH4NO3 is.
I would not be very confident with the perchlorate brother of TEATeN (TEATNP) because despite its higher density and power it would be also much more
sensitive but also likely prone to self decomposition due to the possible switch (low but stil possible transesterification) of the HClO4 from the N
atom to one of the O ester atoms...thus resulting in an unstable mixed nitric perchloric ester.
N(CH2-CH2-ONO2)3.HClO4 <==--> O2ClO-CH2-CH2-N(CH2-CH2-ONO2)2.HNO3
To get back on you molecule, the interraction of H-C#N and CH2=O can lead to HO-CH2-C#N.
The later can be made to react with NH3 (in a 3/1 ratio) to get N(CH2-C#N)3.
Finally N(CH2-C#N)3 can be reduced to N(CH2-CH2-NH2)3 and further reacted with CH2=O (in a 1/3 ratio) to get the structure you proposed.
Finally by interracting I-CH2-CH2-OH with TEA, one should be able to get a quaternary ammonium...
(HO-CH2-CH2-)3N+ I-CH2-CH2-OH --> (HO-CH2-CH2-)4N(+)I(-).
(HO-CH2-CH2-)4N(+)I(-) + AgNO3 --> (HO-CH2-CH2-)4N(+)NO3(-) + AgI(s)
(HO-CH2-CH2-)4N(+)NO3(-) + 4 HNO3 --> (O2NO-CH2-CH2-)4N(+)NO3(-) + 4 H2O
This last compound would be Tetrahydroxyethylammonium nitrate tetranitrate ester or tetrahydroxyethylammonium pentanitrate (THEAPN) (C8H8N6O15).
This must be dense and powerful compound.
[Edited on 17-6-2011 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I am not much concerned about sensitivity due to the perchlorate. Organic amine perchlorate salts are not extremely. It is only when there is
a combination of hydrazine or hydroxylamine with perchlorate ions that the compounds become very sensitive.
It is doubtful that there would be any exchange between perchlorate anions and the nitrate ester, as perchloric acid is much more acidic than nitric
acid outside of aqueous solution.
Interesting idea you had, although I am not completely sure about the hydroxyl group in HOCH2CΞN being replaced with an amino group. Obviously
NH3 does not react with plain alcohols, such as HOCH2CH3. But because of the equilibrium, HOCH2CΞN might work.
Trimethylamine Nitrate is much more acidic than ammonium nitrate, whereas tetramethylammonium nitrate is completely neutral, as the positive charge
gets 'locked' into place on the nitrogen atom, and there is no equilibrium of deprotonation.
I believe you refer to tetraethanolammonium salts.
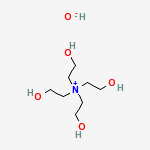
Tetraethanolammonium hydroxide can actually be purchased from specialty chemical companies. It is a white, crystalline solid, used as alkaline
catalyst, as solvent for certain types of dyes, in screen printing of dyes, and in metal-plating solutions.
[Edited on 17-6-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I have made NH2-NH2.HOClO3; it is very hygroscopic and it is quite stable towards heat...and moderately sensitive towards shock when anhydrous, but
when melted and overheated, it is very sensitive and powerfull.
I was speaking of the possible moeity -CH2-O-ClO3 into the molecule.
Even if HOClO3 is much more acidic then HO-NO2 (in water); here there will be almost no water and so on a molecular level transesterification may
occur despite the acidity gap...even if it is 1% or 0.1% level traces would you wish to have a CH3-O-ClO3 brother into a powerfull HE?
Even if not very efficient NH3 do react with alcools, it is one of the first method to make an amine listed in organic chembooks...the only problem is
the selectivity of the method...because you get mono, di and trialkylamines. http://en.wikipedia.org/wiki/Amine
Yes tetraethanolammonium hydroxyde is what I was thinking of and tetraethanolammonium pentanitrate apparently do exists and is listed here  http://www.patentgenius.com/patent/6946042.html http://www.patentgenius.com/patent/6946042.html
Does someone have info about it like density, sensitivity, heat resistance, VOD.
The interesting point is that it would be neutral and so nothing would avoid the making of the perchlorate brother...(O2NO-CH2-CH2-)4N(+)ClO4(-) by a
anion exchange.
[Edited on 17-6-2011 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I meant that perchloric acid is more acidic than nitric acid when no water is present.
When there is water, they have the same acidity.
Can you please provide a reference to anhydrous ammonia reacting with plain alcohols? I thought the two do not react except at elevated temperatures
with a silicoaluminate catalyst. I am not sure how feasible this would be for the home experimenter.
I cannot find any information on hydrazinium perchlorate, but I found this:
"hydroxylamine perchlorate, hygroscopic solid
melting point between 87.5 and 89degC. Decomposes at 120degC.
Drop height value of only 2cm, meaning very sensitive to impact."
I would think NO3[-] [+]N(CH2CH2ONO2]3 would only be somewhat less sensitive than PETN.
[Edited on 17-6-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
|