Random
International Hazard
    
Posts: 1018
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
Sugar experiments
Today I tried to do some experiments with sugar and wanted to try to obtain hydroxymethylfurfural or some other aroma compound.
I took some aluminium foil and put inside a tablespoon of sugar then made something like a retort with sugar inside it. It wasn't 100% closed but some
vapors still have distilled.
Then I placed that retort end into the test tube which was cooled with water and heated that Al retort with candle. Some vapors escaped while some got
into the test tube and I received about 1-2mL of yellow liquid with 1mm caramel colored oil droplet that is insoluble in the remaining liquid. The
liquid has something like caramel smell, but also it has strong strange smell.
Then I left the retort to cool and I have broken it into small pieces and put everything into the jam jar. Then I added like 100-200mL of water, put
the cap on and shook it a lot. The water quickly became very very brown in color and when I shook it the foam was made (like soap), but it quickly
again dissapeared. That brown liquid smells like caramel and my hands too because some of that stuff got on my hands. (I am writing this on the
keyboard and I can still smell very weak smell of caramel from my hands.) This liquid has some hydroxymethylfurfural I think because of the smell.
Now, is there something I can do with this liquid to isolate some aroma compound? I was thinking since 5-HMF is highly soluble I would just filter
that solution from carbon and aluminium particles. 5-HMF should form bisulfite adduct because it is aldehyde and alcohol at the same time, if there is
furfural present it should make it too. Just I am not sure, are their adducts insoluble in water?
By the way, what could be that small brown oil droplet that is denser than water in the test tube?
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
The browning of sugar is also an interesting phenomena. Essentially the sugar becomes dehydrated, forming an additional aldehyde group on the
molecule.
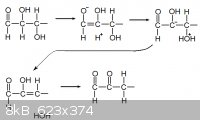
http://class.fst.ohio-state.edu/fst605/605p/Maillard.pdf
(see slide 3)
Here is the reaction mechanism typed if the picture becomes lost at a later time:
O=CH-CH(OH)-CH(OH)-R
HO-CH=C(OH)-CH(OH)-R
[-]O-CH=C(OH)-CH(OH2[+])-R
O=C-CH(OH)=CH-R H2O
O=C-C(=O)-CH2-R H2O
[Edited on 18-3-2012 by AndersHoveland]
|
|
|
Random
International Hazard
    
Posts: 1018
Registered: 7-5-2010
Location: In ur closet
Member Is Offline
Mood: Energetic
|
|
So basically, aldehydes are formed from sugars. Interesting.
Does someone have information about isolation of different compounds from that dry distillation? I am sure I made a small amount of some compound and
it smelled strongly of caramel, I would like to isolate the compound.
|
|
|