White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Chemical stability of vanadium II oxide
Vanadium II oxide is an interesting non-stoichiometric vanadium oxide that conducts electricity.
I would like to get my hands on this vanadium oxide, but can't find any info on how to make it and I don't even know if this substance is stable at
room temperature and in the presence of oxygen and water. There is a significant lack of information on the chemical properties of this compound.
Is vanadium monoxide stable at room temperature and in the presence of oxygen and water?
And if so, how would I go about making it from ubiquitous vanadium pentoxide?
Does anyone have links to literature on the subject?
I have checked wikipedia, and it says that VO is stable at room temperature, but I don't trust wikipedia.
Thanks in advance.
|
|
|
Bitburger
Harmless

Posts: 41
Registered: 23-7-2011
Location: Belgium
Member Is Offline
Mood: Experimental
|
|
VO is a stable grey, black oxide with a mp.1790°C
The best way to make it is just a acid reduction with Zn or Fe. V2+ solutions are violet, V3+ is the green one. But the issue is that the violet
solution turns back green on heating.
VO is soluble in acids.
To be honest I have never try this reduction. So, I don't know much more about it.
[Edited on 7-8-2011 by Bitburger]
Good to be wrong
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
V(II)O will decompose water, releasing hydrogen; in strongly acid solutions V(II) can be made and will exist for short periods of time. I''ll try to
find a synth for it, check the Inorganic Synthesis volumes for one.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
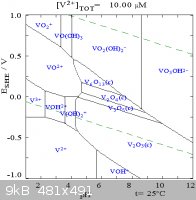
V(II) oxide slowly reacts with air, and will ignite if warmed in air.
The Pourbaix diagram for aqueous vanadium shows the problem in working with V(II). The lower dashed line is the reduction of water to H2(g).
-----------------------------------------------------
38. HYPOVANADOUS OXIDE AND VANADIUM
OXYTRICHLORIDE
V2O5 + 3H2 -->. V2O2 + 3H2O
V2O2 + 3C12 --> 2VOCl,
SUBMITTED BY F. E. BROWN*A ND F. A. GRIFFITTSt
CHECKED BY G. B. HEISIG$A AN D L. A. ENBER
A. HYPOVANADOUS OXIDE
A pyrex tube 100 em. long and about 2.5 em. in diameter
is filled to one-third of its capacity with vanadium pentoxide.
After the air has been displaced with hydrogen, the
tube and its contents are heated to a dull-red heat. A slow
stream of hydrogen is allowed to flow until the mixture in
the tube becomes black. Heating is then discontinued, but
hydrogen is passed through the tube until its contents are
cool.
|
|
|
sternman318
Hazard to Others
  
Posts: 121
Registered: 21-4-2011
Member Is Offline
Mood: No Mood
|
|
Misread OP- Ignore this
[Edited on 8-8-2011 by sternman318]
|
|
|