yoyoils
Harmless

Posts: 22
Registered: 6-8-2011
Member Is Offline
Mood: positive
|
|
Enthalpy question
my textbook is telling me
(sum of strengths of bonds broken) - (sum of strengths of bonds formed) = 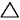 Ho Ho
so naturally I look up bond strength charts to find there's just bond dissociation and bond energies if I'm correct. It's kinda hard to find out which
I should be using but here's the practice problem.
CH4 + 2 O2 ----> CO2 + 2 H2O(liq) answer is -213kcal mol-1
except somehow I'm getting -198kcal mol
I got my answer trying to use http://www.cem.msu.edu/~reusch/OrgPage/bndenrgy.htm
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
This belongs in beginners.
You'll need to detail your calculation.
This is a combustion enthalpy. At 298 K, the Standard Heat of Combustion can be found by adding the up the Standard Heats of Formation of compounds on
the right and subtract from it the Standard Heats of Formation of compounds on the left. The Standard Heats of Formation can usually be found in NIST
Webbook.
|
|
|
yoyoils
Harmless

Posts: 22
Registered: 6-8-2011
Member Is Offline
Mood: positive
|
|
Is this standard heats of formation? http://chemistry.about.com/od/thermodynamics/a/Heats-Of-Form...
I actually learned all of this a few months back but obviously didn't remember it so now im just relearning but i can't find my original resource
(chart) that helped me get right answers lol
My book says "Subtract formed bond strength from that of the bonds broken".
so I would need something telling me formed bond strengths and broken bond strengths to add them each together by kcal mol-1 and subtract the totals
after that I know you get a negative number means exothermic (outputs energy) and positive endothermic (requiring energy input)
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Calculating an enthalpy change using energies for making and breaking bonds will only be approximate, usually. Use of enthalpies of formation will
give the right answer.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
yoyoils
Harmless

Posts: 22
Registered: 6-8-2011
Member Is Offline
Mood: positive
|
|
Alright, sorry this is about as far as I understand after some review to get back to what I learned before.
1. Calculate the approximate enthalpy change for the combustion of methane:
CH4 + 2O2 ---> 2H20 + CO2
2. Relevant equations
CH4 + 2O2 ---> 2H20 + CO2
enthalpy= D(reactants) - D(products)
D= bond dissociation energies
Bond Dissociation energy (KJ/MOL)
C-C 350
C=C 611
C-H 410
C-O 350
C=O 732
O-O 180
O=O 498
H-O 460
3. The attempt at a solution
ENTHALPY= D(reactants)-D(products)
[1640+(2*498)]-[(2*920)-1464]= -668 kJ
the hole problem here is my book "Organic chemistry structure and function fourth edition" says the answer is -213 kcal mol-1
|
|
|
yoyoils
Harmless

Posts: 22
Registered: 6-8-2011
Member Is Offline
Mood: positive
|
|
I'm sorry for doubleposting but do I have people stumped? lol
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Like I said, this is much easier and more accurately solved by looking up the Heat of Formation DHf of reactants and reaction products, then basically
add them up according to Hess Law.
In your case:
DHcombusion = 2 DHf, H2O + DHf, CO2 - DHf, CH4 (Dhf, O2 = 0)
The HoF values at 298 K (Standard Heat of Formation) can be found in NIST Webbook, formula search:
http://webbook.nist.gov/chemistry/form-ser.html
Tick the box 'gas phase' or 'condensed phase' to access HoF of a given substance, provided NIST lists it.
Using bond energies is approxinate only, because they depend on the entire molecule: the same bond doesn't necessarily have the same energy in two
different molecules. HoF on the other hand is an experimentally determined value (although usually indirectly).
We're not easily stumped at SM! 
|
|
|
yoyoils
Harmless

Posts: 22
Registered: 6-8-2011
Member Is Offline
Mood: positive
|
|
Oh, wow lol
|
|
|