ZHANGNIUBI
Harmless

Posts: 28
Registered: 5-5-2011
Member Is Offline
Mood: No Mood
|
|
Need some help about α-Bromination
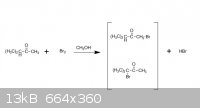
Hey,guys, I m in trouble with this problem:
when using CH3OH as solvent and Br2, the major production is the first one, and when CCl4 as solvent and Br2, the major production is the second one.
I cant figure out why.
Thank you
[Edited on 17-9-2011 by ZHANGNIUBI]
|
|
|
ZHANGNIUBI
Harmless

Posts: 28
Registered: 5-5-2011
Member Is Offline
Mood: No Mood
|
|
That may be about the differences between kinectic production and thermodynamic production. But what is the real reason?
|
|
|
francis
Hazard to Self
 
Posts: 72
Registered: 1-4-2011
Location: Australia
Member Is Offline
Mood: No Mood
|
|
Will you post the picture again please? The link doesn't seem to work.
Or just write the names of the products if you can.
The reason is because of the difference in solvent polarity: methanol is a polar solvent, and CCl4 is a nonpolar solvent.
If you are talking about alpha-bromination of a carbonyl, the enolate intermediate will be stabilised by a polar solvent. I don't know if an enolate
forms without the presence of acid or base.
If you post the image again, it would be helpful
[Edited on 17-9-2011 by francis]
|
|
|
drago57
Harmless

Posts: 16
Registered: 13-8-2011
Member Is Offline
Mood: No Mood
|
|
Are you excluding light from your reactions? Bear in mind even a fluorescent strip light will be enough to photolyse Br2
I'm wondering if your reaction in CCl4 is going via a radical mechanism whilst the rection in MeOH is going via the enolate intermediate. Try them
again with your flasks wrapped in foil and see what you get out of that
|
|
|
ZHANGNIUBI
Harmless

Posts: 28
Registered: 5-5-2011
Member Is Offline
Mood: No Mood
|
|
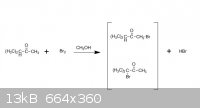
thank you, guys,here is the document. And the the light was not mentioned..it seems like it s about how solvent control the major production..
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ZHANGNIUBI  | | when using CH3OH as solvent and Br2, the major production is the first one, and when CCl4 as solvent and Br2, the major production is the second one.
|
In the first post you make it sound like you ask about your own experimental results, but don't provide us the experimental and analytical data. In
the later post you make it sound like you are asking about a literature example, but you don't provide the reference. In any case you are not giving
sufficient data!
|
|
|