Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
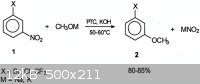
1-bromo-3-methoxybenzene
Hi all,
1-bromo-3-methoxybenzene, aka, 3-bromoanisole is my target compound for a synthesis.
Now there is one big problem with this synthesis is anisole is ortho and para directing and so the direct bromination of anisole with bromine would
yield the wrong isomer.
Joseph Zilberman et al has produced a paper in which meta substitued methoxy halobenzene can be produced from 3-halonitrobenzene but even this is a
challenge to make. The paper is here:
http://pubs.acs.org/doi/abs/10.1021/op020058t
Can anybody come up with any other practical synthetical methods of producing this compound? I need around 10g so the reaction must be able to be
scaled acordingly.
Thanks in advance
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
I had an interest in this compound a while ago, and while I never completed the synthesis I had good results with the procedures.
I will recomend you look into an earlyer thread which deals with many of the same problems: http://www.sciencemadness.org/talk/viewthread.php?tid=15521#...
Anyway there are a few papers in that thread which are full of valuable information.
Good luck
|
|
|
francis
Hazard to Self
 
Posts: 72
Registered: 1-4-2011
Location: Australia
Member Is Offline
Mood: No Mood
|
|
This paper advises reaction of 3-bromophenol with methyl bromide and potassium carbonate in DMF, reflux for 6 hours.
An Improved Synthesis of Triarylphosphines
I don't have access to the paper though: I got the information from SciFinder which gives a summary of the reaction and the conditions.
This PDF document suggests it can be obtained from 3-methoxyaniline via a Sandemeyer reaction (see page 5):
http://www.dtic.mil/cgi-bin/GetTRDoc?Location=U2&doc=Get...
Here is a reaction scheme for 3-bromophenol from nitrobenzene, then maybe from 3-bromophenol you could use the reaction in the first paper.
http://de.wikipedia.org/wiki/Datei:Synthesis_3-Bromophenol.s...
[Edited on 11-10-2011 by francis]
|
|
|
Dr.Bob
International Hazard
    
Posts: 2659
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Since the 3-bromophenol is 3 times more expensive than the 3-bromoanisole, I would think that the phenol is likely made from the anisole,
commercially. But that may not be relevant. It mostly depends on if you can get 3-bromophenol. If so, it is easy enough to methylate is most
electrophilic methyl sources.
The other routes that I find are all old, but show both 3-methoxyaniline and 3-bromoaniline being converted to the 3-bromoanisole.
3-methoxyaniline being converted to the 3-bromoanisole(HBr and Sodium nitrite are listed): Berti; Da Settimo. Annali di Chimica (Rome, Italy),
1959 , vol. 49, p. 1237,1248
3-bromoaniline being converted to the 3-bromoanisole: Multi-step reaction with 2 steps:
1: aqueous H2SO4 / Diazotization.Kochen der Diazoniumsalz-Loesung
2: aqueous NaOH
Natelson; Gottfried. Journal of the American Chemical Society, 1939 , vol. 61, p. 1001
or
Multi-step reaction with 2 steps
1: aqueous H2SO4 / Diazotization.Kochen der Diazoniumsalz-Loesung
2: aqueous KOH
Hewett. Journal of the Chemical Society, 1936 , p. 50
|
|
|
SmashGlass
Hazard to Self
 
Posts: 52
Registered: 25-1-2011
Location: Scandinavia
Member Is Offline
Mood: No Mood
|
|
I was thinking of a different approach.
Bromination of benzenesulfonic acid to the 3-Br.
Then formation of the methyoxy via dimethylsulfate at refulx, after initial treatment with NaOH in water.
Dimethyl sulfate is not the nicest reagent on the planet, but does what it is supposed to.
Any opinions on this approach? Pro's cons...
The other way from the above mentioned starting materials would be a longer approach:
reduction of nitro, diazotization, formation of the phenol and following through to the methoxy.
Alternatively the phenol may possibly be skipped via a similar dimethlysulfate pathway.
The desired product does sell for about £30 for 25g as well.
But if you are just out for the challenge then I guess you can synthesize it too...
If it ain't broke don't fix it....
Now where are my screwdrivers?  |
|
|