| Pages:
1
..
15
16
17
18
19
..
25 |
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
I'm curious about this mentioned compound on the bottom of this page: https://sites.google.com/site/ecpreparation/nickel-hydrazini...
The author mentions a clathrate compound comprising of up to 10% AgN3 and the other 90% NHN. how do you think one would go about making such a
clathrate? or is it more likely to be just a double salt of the two and not a true clathrate? it says the mixture is co-crystallized from a solvent,
which is not mentioned.
Has anybody here heard of this compound before or had experience with it? it does sound interesting.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by a nitrogen rich explosive  | I have just synthesised 5 grams of zinc tetramine permanganate through the reaction of KMnO4 with an excess of a solution of 3% ammonia, zinc oxide
and ammonium chloride. The temperature must be kept to a minimum without freezing, because the resulting product (Zn(NH3)4)(MnO4)2 decomposes with
H2O. It is therefore unusable, with a shelf life of 1-2 hours, due to moisture in the air.
However, I was thinking: would it be possible to synthesise something like nickel tetramine permanganate (Ni(NH3)4)(MnO4)2 or cobalt/silver tetramine
persulfate/nitrate? It would, but I don't know how energetic they'd be.
Any ideas? |
Why posting the very same kind of questions in various treads of the energetic materials?
here and here too
If it is so sensitive to moist air, how do you cope with the 97% water content of your NH3?
No it would not!
If it does, Nickel will make a hexaamino complex and not a tetramino; so does Cobalt.
Silver will at best make a diamino complex.
[Edited on 6-4-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by NeonPulse  | I'm curious about this mentioned compound on the bottom of this page: https://sites.google.com/site/ecpreparation/nickel-hydrazini...
The author mentions a clathrate compound comprising of up to 10% AgN3 and the other 90% NHN. how do you think one would go about making such a
clathrate? or is it more likely to be just a double salt of the two and not a true clathrate? it says the mixture is co-crystallized from a solvent,
which is not mentioned.
Has anybody here heard of this compound before or had experience with it? it does sound interesting. |
I think it is a website from user AndersHoveland, sadly he hasn't post here since end 2013 and didn't show here since 2014.
Sadly also the website and its various clones, are a collection of ideas of AH himself and copy paste from various sources but usually without
references...and just like its author a bit confuse and missing coherence/structure.
Anders was used to post weird receipts from obscure or unreferenced sources...
I even noticed in your link my own receipt for NiHN from mixing of alcoholic hydrazine and Ni(NO3)2 solutions, but of course omitting the source (me
and this forum)... 
For the rest, any mixing of NHN with a compatible unsoluble sensitive primary will make a symbiotic mix beneficial to both compounds...lowering the
sensitivity of the primary and sensitizing NHN making it more reliable for D2D; NHN will give its power and high VOD.
I have used silver nitrato acetylide complex.
I guess AgN3 would be even better.
I don't know how it would be done as a cocrystalisate...it all depends if Ni(N3)2 is unsoluble or not and how it will react with
hydrazine...Ni(N2H4)3(N3)2 may form if I recall correctly.
Maybe mixing Ni(N2H4)3(NO3)2 with AgN3 in NH4OH will do the trick, but if I recall wel from my own experience ammonia destroys the hydrazino
complex...but maybe I have mistaken solubilisation with destruction...because in theory Ni(N2H4)3(NO3)2 should be in equilibrium with Ni(NH3)6(NO3)2
and if the system is open while NH3 evaporates faster than N2H4, then the initial unsoluble complex will redeposit aside with AgN3.
[Edited on 6-4-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
Recently I made two batches of NHN. The first was blue and very impure, I think EtOH made Calcium Hydroxide precipitate along the NHN. The second
batch, this time without EtOH, seems to be fairly pure and was washed 2 times with water. But I'm turning crazy trying to detonate it.
The only way I get a DDT was heating it in a 5mm diameter copper pipe, both sides sealed. It requires strong confination and behaves more like a
nitrated polyol.
What's the best way to detonate it? Maybe flash powder? I could try to sensitize it with another primary, but I don't wanna mess with azides. At the
moment I only could try mixing it with DDNP or DNPH.
|
|
|
Eosin Y
Banned troll
 
Posts: 83
Registered: 8-5-2016
Location: Eton College science department
Member Is Offline
Mood: Aga needs to cool his heels
|
|
Detonation with some kind of super flash might be an idea.
Copper tetraammine azide/copper hydrazine azide might be interesting. However, I doubt that CuTA would be stable enough to not leak ammonia.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Eosin Y  | Detonation with some kind of super flash might be an idea.
Copper tetraammine azide/copper hydrazine azide might be interesting. However, I doubt that CuTA would be stable enough to not leak ammonia.
|
How do you do to make Cu azide hydrazino complex?
And thus avoid the decomposition of the hydrazine (reducer) by the Cu(2+) oxydant?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Eosin Y again  | | I thought that the Cu2+ ion was only repellant to the O2 ion, and that copper hydrazino azide would stay together like copper peroxide.
|
Why "Eosin Y" and now "Eosin Y again" ?
Don't think, verify first!
Could you elaborate?
1°) Cu(2+) repellant to O2?
2°) O2 ion?
3°) Cu peroxide? How do you do this and stabilize it?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Eosin Y again
Banned troll

Posts: 13
Registered: 14-5-2016
Member Is Offline
Mood: No Mood
|
|
I'm surprised that you didn't notice the quad-trainwreck with me, Bert, Aga, and Blogfast that ended up in Bert going off in a rage and removing Eosin
Y's posting priviledges.
https://en.wikipedia.org/wiki/Copper_peroxide
Schweizer's reagent and H2O2.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Rage was not involved. My mother was a psychologist, I only feel sadness when I run into the damaged people who act out on the net due to their
mental health issues . And a desire to get them into a proper treatment regime where they may learn to act appropriately in public, perhaps eventually
leading a normal life.
Why didn't you even bother to mask your ip while running the troll accounts this time? Before, you at least TRIED to hide. Using
enormousdick@hotmail.com for a sign up email address, is it a cry for help, or merely the arrogance of a sociopath.
octonitrocubane, a nitrogen rich explosive, eosin-y and eosin-y again. Part of your psychopathology requires you to let others know that you have had
them on and wasted their time? Or it's no fun to pretend to be an English schoolboy until you are caught?
[Edited on 15-5-2016 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I had a good intuition/feeling that those 4 pseudos were in fact the same person  . .
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
So following your link CuO2 is just like I wrote unstable.
But CuO2 will have much gentle consequences than your putative Cu(N2H4)2(N3)2 because this last would be explosive.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
KesterDraconis
Hazard to Self
 
Posts: 78
Registered: 27-3-2015
Member Is Offline
Mood: No Mood
|
|
So I tried the synthesis of NHN and I got a blue precipitate. However it did not change lilac purple, but instead stayed blue, even when I agitated
the solution a bit and added more of the nickle nitrate solution. I'm letting it dry now, but its still not changing. Is there a reason for this
odd/out of place color?
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1333
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
NHN
Will be better added hydrazine, not NiNO3. Small amount hydrazine = blue. Surplus Hydrazine = lila. If you has pure materials. If you has Hydrazine
and NiNO3, is impossible any another reaction. Arises NHN. In a small surplus hydrazine hydrate. ...LL..
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Yes LL is right!
When adding Ni(NO3)2 to N2H5OH at first without agitation you get locally a blue flocculating precipitate (where the green concentrated drop of Ni(2+)
crashes to the bottom of the beaker...
When you mix it, it turns lilac pink with the surrounding exces of N2H5OH.
--> You had too much Ni(2+) and too less/not enough N2H4.
Meaning you have transition/uncomplete complexation like Ni(N2H4)(NO3)2, Ni(N2H4)2(NO3)2 and maybe traces of Ni(N2H4)3(NO3)2 ...
But not plain Ni(N2H4)3(NO3)2.
[Edited on 14-6-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
KesterDraconis
Hazard to Self
 
Posts: 78
Registered: 27-3-2015
Member Is Offline
Mood: No Mood
|
|
Ah, this actually makes sense, on a second attempt I noticed it was lilac purple for a bit, then turned blue and stayed that way. When this
precipitate was dried, I found myself with a substance that did not burn very well, but surprisingly made a nearly unnoticeable popping sound when
confined. I will try again tomorrow and do the reverse by adding the hydrazine to the nickle nitrate, and add it in greater quantity (though I'm
trying to keep things very small here, for obvious reasons).
I will report back here with results in a few days, thanks!
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1333
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
NHN
Well, here is difference between not complete reaction (1) and result complete reaction with surplus hydrazine (2) Use HH 80%....LL...

|
|
|
KesterDraconis
Hazard to Self
 
Posts: 78
Registered: 27-3-2015
Member Is Offline
Mood: No Mood
|
|
I would like to thank all the members here for all the contributions to my successful synthesis of NHN, particularly PZ and LL here. It really is a
wonderful color change (one of my favorite colors is lilac purple actually), and I was very impresses and pleased with the explosives characteristics.
If I were one to set off large charged (which I am not, I prefer tiny bits of smaller things, for experimentation) I would probably use this, low
friction and impact sensitivity, but high enough flame sensitivity to be useful in the amounts necessary to act as a primary. Very good all around it
seems.
Thanks again for the help guys, I can finally check this off my list of "things I would like to synthesize and experiment with". (about time too, its
been on the list a year!)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1333
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
NHN
Well, NHN is beauty and even has nice smell. Thus my experience. Pretty silent a flame. As nitrocellulose, but still speeding. Until detonation.
(huhaha). But my experience is difficult transfer to fully velocity on 7000 m/s. At 1.7g /cm3. However, is it the way, it is development. .. ...LL ...LL
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Yes!
Unlike other primaries NiHN is not reliable in too low/tiny quantities...this means that the D2D transition is not effective below a certain amount
that is quite large vs real primaries.
NiHN doesn't detonate unconfined except in even larger quantities displaying enough self-confinement.
When wrapped in Al foil (confined) and heated by a flame it deflagrates/detonates with a very loud report and huge fire ball.
When 100 mg of such wrapped stuff is trown into a camp fire, after a second or more, it makes a fire ball of about 40 cm diameter and trows
incandescent charcoal all arround a few meters away!   
I use the co-mix of SADS/SANC (silver acetylide double salt, silver acetylide nitrato complex) with NiHN to circumvent this lack of reliability (mix
SADS/NiHN 1/3 to 1/4 by weight).
Since both are unsoluble clayish stuffs (white creamy and lilac pink...the resulting mix is paler lilac pink).
This is synergetic co-mix because SADS offers immediate D2D and flame sensitivity even in minute amount, while NiHN offers its brisance, higher VOD,
and energy output.  
I'm more than eager to follow the results of Laboratory of Liptakov about the improvement of NiHN D2D without help of a conventional primary (NPD
detonator) like/based on the now famous Berta kind he designed/invented...    
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1333
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
NHN
Well, well... Thanks. Was making next 3 tests, about NiHN. Without SADS mixing, but with partially using copper hexamine perchlorate, respectively was
used this mix: Tetraamine Copper Perchlorate 88% + 6% hexamine + 6% NH4ClO4. Boiled together by 100 Celsius, evaporated by 60 C. The Grain 1x1mm. For
NiHN (DDT segments) was used this mix: NiHN 94% nitrocellulose 4% magnesium 2%. Nitrocellulose is necessary for making aglomerate 1x1 mm, for best
function of detonation transfer. (DDT) Magnesium should by increase DDT also.
Next. First attempt (red 1) was used for output segment pure NiHN 0,3 grams, it see on scheme. Other segments are NiHN mix described. From result is
clearly, that NiHN was detonated, or partially detonated. This is the sharp edge around in steel 2mm thickness. Thus DDT worked. But not much power.
But good detonation (power) for using next secondary segment ETN.
Second attempt, (red 2) was used same output segment from NiHN (0,3 pure grams), but other material in next segments was CHP (described up) . Again
from aglomerate basically large by 1x1 mm. It see, that energy was bigger. It maybe was a full detonation NiHN.
For comparsion was (red 3) using only CHP for all segments. Thus system Berta on Copper hexamine perchlorate (CHP) based. All attempts are not in
system NPED, because NiHN and CHP are primary substance. But both compounds has much more safety, than HMTD and relatives. All others important is
in scheme, how I hope. ...LL...

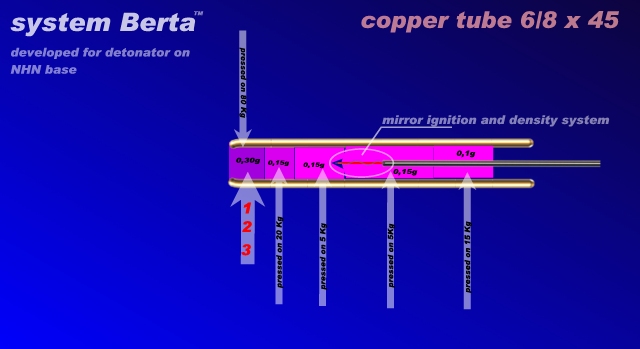
[Edited on 22-6-2016 by Laboratory of Liptakov]
|
|
|
KesterDraconis
Hazard to Self
 
Posts: 78
Registered: 27-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PHILOU Zrealone  |
I use the co-mix of SADS/SANC (silver acetylide double salt, silver acetylide nitrato complex) with NiHN to circumvent this lack of reliability (mix
SADS/NiHN 1/3 to 1/4 by weight).
Since both are unsoluble clayish stuffs (white creamy and lilac pink...the resulting mix is paler lilac pink).
This is synergetic co-mix because SADS offers immediate D2D and flame sensitivity even in minute amount, while NiHN offers its brisance, higher VOD,
and energy output.   |
I actually like this idea, since it helps to get rid of the one con of SADS, which is its low power.  If only I could make some more to experiment with, but alas, I used up the last of my hydrazine, and have no time to
make more before my university summer classes begin. I will save it for Christmas break I think. If only I could make some more to experiment with, but alas, I used up the last of my hydrazine, and have no time to
make more before my university summer classes begin. I will save it for Christmas break I think. 
I also appreciated the experiments LL! I find it odd that the NHN doesn't seem to be producing very much power and detonating fully. I noticed the
batch I made was very very reluctant to detonate at all, but I'm still surprised that it doesn't detonate in the manner you are using it. Perhaps it
would simply be best as you said, to use something like number 2, and an ETN secondary. You could then use that as your booster for larger charges,
and still have some of the safety of the NHN.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
@Laboratory of Liptakov please edit and limit image width to 650 pixels because it destroys the page format for 4X5 aspect ratio monitors, when larger
images are placed inline.
Thanks for the image resizing. It looks better now.
NHN is critical diameter sensitive for DDT and should transition upward in velocity with a significant jump in brisance at a larger charge diameter,
and I'll have to check my references to confirm but IIRC it was in the area of 9-10 mm. It isn't uncommon for slow self accellerating primary
explosives, and there are several others that show the same sluggishness, but above a certain diameter and above a certain critical mass, which for
some might even be several grams ....will get the job done when they do go high order.
It could be a different method of synthesis could produce a more dense crystalline form of NHN that would perform better.
I have wondered if instead of using hydrazine hydrate, maybe try hydrazine nitrate in presence of ammonia as the hydrazine value.
Possibly just take hydrazine sulfate and render more soluble as the neutral dihydrazine salt by adding an equivalent of ammonia, then add a solution
of calcium nitrate to precipitate the sulfate as calcium sulfate and filter out leaving a solution of hydrazine nitrate. Simultaneously add gradually
nickel nitrate and additional ammonia in separate streams dropwise with stirring and low heating. Such an approach might allow for crystal growth of
the low solubility NHN, if it will form under such conditions as I am thinking it will form by a more gradual and slower reaction.
It may be possible also to simultaneously form and introduce some azide content by utilizing a nitrite of nickel in mixture with the nickel nitrate,
possibly forming the nickel nitrite by adding sodium nitrite to react with some of the nickel nitrate solution.
In the alternative, putting an azide like sodium azide in solution with the ammonia to be added dropwise separately, parallel with the nickel nitrate
solution, to the ammoniated hydrazine nitrate solution. Possibly some azide content in the NHN could improve its self accelleration and small
diameter performance.
[Edited on 6/22/2016 by Rosco Bodine]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1333
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
NHN
If you have ETN and NiHN is easily do it prepare DDT mix: ETN 70% + NiNH 25% + 5% nitrocellulose for better agglomerates 1x1 mm. All is mixed in
acetone, a few a drops. Arises paste, through the sieve do it agglomerate and slowly dry process evaporate acetone by 40 Celsius. This deflagration -
detonation mix is filled according scheme. Only output segment is from pure ETN. His Pressing can be higher cca 100 Kg on 6mm diameter. This
construction worked perfectly always. Ratio ETN-NiHN for DDT segments can be pretty free arbitrary. For example even 50 : 50. And worked it.
Necessary is content (4 - 7%) nitrocellulose, commerce grade, thus with content nitrogen 12 - 12,6 %. I not recommend homemade NC, because is usually
with residuum acid... ...LL ...LL
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
ETN has a good but limited shelf life ~4-5 years and is not so good at warmer temperatures, so for long term storage devices ETN is not viable. You
have an expiration date on anything in which you use ETN. In the alternative, there are other more stable materials for which the shelf life is
indefinite, perhaps even hundreds of years. PETN would be better for long storage, and likely p-DDNP would be better also, though the power output
would be reduced compared to ETN or PETN....as an OTC material p-DDNP could have advantages over ETN if there was need for long storage endurance.
[Edited on 6/22/2016 by Rosco Bodine]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1333
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: cool.gif
|
|
NHN
In system Berta of course worked also PETN. I had to write directly together upstairs, sorry. One different is only this, that content of nickel
hydrazine nitrate must be (in DDT mix) between 50 - 40 %. Lower content shows on some failed. At 20% for example. All others parameters are same,
prepare, pressing according the scheme here upstairs. ... ...LL ...LL
|
|
|
| Pages:
1
..
15
16
17
18
19
..
25 |