| Pages:
1
..
31
32
33
34
35
..
37 |
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
Pure "DPPP"
Damn, somehow got it to submit accidentally...anyway editing in my post now....
[Edited on 7-2-2005 by Chris The Great]
So, I filtered out my "DPPP" last week, and it was dry enough to do some tests on this weekend.
Filtering was somewhat problematic for me, I lost alot of the product from the A batch.
The A mixture was poured into the filter, and distilled water was used to get the rest of the crystals out of the jar. A small amount of sodium
bicarbonate solution was poured onto it, however there was no acidity, so I stopped pouring it and rinsed it again with distilled water. Then, it was
rinsed with methanol, which caused about a third of the product to go through the filter! I was somewhat upset, so rinsed again with distilled water
and then with isopropyl alcohol (99%). The isopropyl went through the filter very slowly however it did not take a bunch of crystals with it as the
methanol did. I squeezed the rest of the isopropyl alcohol out of the filter by hand and put it in a small dessicator.
The B mix was dumped into the filter and distilled water was used to get the rest of the crystals out of the jar. A large amount of sodium
bicarbonate solution was required to nuetralize, and it foamed a large amount during nuetralization. After the product stopped foaming when sodium
bicarbonate solution was added, I rinsed it with distilled water and then 99% isopropyl alcohol. After most of the alcohol had gone through the
filter I squeezed out the rest by hand and put it into another dessicator.
This weekend I had time again, so I went out to check on the "DPPP". It has dried completely in the dessicator, and both the A and B mix
seemed to be exactly the same thing. A few deflagration tests also gave that impression.
About 1 gram of "DPPP" in the form of many small chunks was ignited on a spoon, with a very nice think black smoke ring being formed. I
then deflagrated ~1 gram of each the A and B mixtures on a piece of wood. Both burned very quickly, seemingly more quickly then AP, with think black
smoke being formed each time.
A then took a pill sized piece, also approximatly 1 gram, perhaps a very small amount more, and had it DDT with a very large amount of force. I had
put in hearing protection for these tests, as I didn't know exactly how easily it was to detonate, so my ears only had a very slight ringing that
quickly faded away. Although it was very loud, and I felt a substantial shockwave hit my hand which was in a leather glove about 4 inches away, there
was only a small 1cm dent on the soft particle board. No smoke was produced, and the distinctive smell of detonating AP was not smelt.
I detonated a small <0.5 gram AP initiator I made to test, and the smell was very strong, indicating that what I have was definatly not AP.
I then tried dissolving it in toluene to check the moisture content, as Rosco had found his full of water. About 3 grams where dissolved in 25mL
toluene, and absolutly no water was seen. So, whatever it is, it's pretty pure.
I am hopefully going to try peroxidizing my precursor prepared with sulfuric acid instead of hydrochloric acid tommorrow.
[Edited on 7-2-2005 by Chris The Great]
|
|
|
Atom
Harmless

Posts: 15
Registered: 1-1-2005
Member Is Offline
Mood: No Mood
|
|
Yeah I just tried to make some but it failed. I read you can make Phorone when using sulfiric acid 96 %. So I tried to make some but it had a runaway
of some sort. Theres this distinct smell in the whole house now. Does anyonen know what happenend. By the way I used 5 ml SA and 5 ml acetone. With no
cooling.
Hehe uhm I just made it with cooling and everything works just fine.
[Edited on 7-2-2005 by Atom]
|
|
|
Mumbles
Hazard to Others
  
Posts: 436
Registered: 12-3-2003
Location: US
Member Is Offline
Mood: Procrastinating
|
|
No one is 100% sure on the phorone synthesis as of yet. What you made mostly is probably mesitylene. If the liquid turned orange/red/black it is
mesitylene at least party. The proceedure that you took is the exact one I take for mesitylene when I need it in a hurry.
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
Wow, didn't you read my post about using 96%+ H2SO4 sulfuric? I had mine explode using less acid to acetone ratio than you.
I found 14mL H2SO4 for 55mL acetone worked well, the reaction sustains itself to completion in about ten minutes, when the jar is sealed and mostly
empty. This allows the acetone vapour to reflux by condensing on the sides of the jar. In this case it was a 250mL jar, I wouldn't try putting
any more liquid in or the pressure may rise too quickly and too high.
I am going to try peroxydizing my mixture soon, I haven't had a chance today, but hopefully tommorrow, to confirm that the method using sulfuric
acid works.
Also, I recently aquired approximatly 25 to 30 pounds of lead. While a trauzl block typically is 165 pounds or so, I can probably make a smaller one
and test some explosives with well known block test ratings, and see what I get with this. While the results will definatly not be conclusive, it
might establish a ballpark figure.
I can see problems however, with such a "small" block I would need to use small amounts of explosives, so incomplete detonation may occur.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Hot Toluene is excellent recrystallization solvent
When toluene is heated in a sealed jar and then the crude AP / DPPP is is added ,
it literally will form a syrup which is two thirds or more by weight the dissolved organic peroxide . Any water trapped in the fluffy crude crystals
separates and rises to the top of the hot solution where it can be decanted or ladled off . If the hot saturated toluene solution is allowed to cool
slowly while being stirred slowly on a magnetic stirrer , gritty anhydrous crystals of much improved density gradually precipitate . I tried adding
denatured alcohol to the toluene solution hoping that this would speed the precipitation , but this was unsuccessful because the alcohol / toluene
mixture exhibited a cosolvent effect and no organic peroxide precipitated . The alcohol did form an upper layer containing the water in solution ,
along with a fair amount of the organic peroxide also in solution . Upon evaporation , this separated moist alcoholic solution also deposited gritty
crystals of much improved density .
|
|
|
Atom
Harmless

Posts: 15
Registered: 1-1-2005
Member Is Offline
Mood: No Mood
|
|
What do you use mesitylene for then?
'Cause I just tried to peroxidise this but it didnt work. There were just these two seperate layers and they wouldn't mix properly. One
turned green and the other one just stayed oily sticking to everything it could.
And I thought mesitylene is colourless but this has a deepred colour.
[Edited on 8-2-2005 by Atom]
|
|
|
Quince
National Hazard
   
Posts: 773
Registered: 31-1-2005
Location: Vancouver, BC
Member Is Offline
Mood: No Mood
|
|
So, is this thread dead or what? And what happened to Rosco, he hasn't been on the board for like three days? Maybe some accident in the lab?

\"One of the surest signs of Conrad\'s genius is that women dislike his books.\" --George Orwell
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Still around , just not much time for experiments lately . Been too busy otherwise . The hot toluene recrystallization of a mixed product produced
what appears to be a single material . From 100 ml of toluene heated
and then saturated while hot with the organic peroxide , 75 grams of the anhydrous crystals were obtained when the solution cooled . The solution was
stirred slowly using a magnetic stirrer during the several hours it was cooling ,
and gritty cystals of improved density were obtained , which dried much more quickly than crystals from aqueous solution .
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
Hello!
A few hints: color changes are a bad indicator for purity or a specific product in org. chem....for example: you can have a 99% pure product which is
colourless in the pure state and it can eventually be anything from red - yellow- orange or black -coloured in your finished batch.
I´d propose adding 1/2 g of fine activated charcoal to decolourize the solution and stirring/warming for 1-2 hours.
Another thing: somebody mentioned using hot toluene as recrystallizing solvent.....isnt that hazardous? - toluene boils at ~110°C....acetone
peroxides (which are most likely major products when following the mackowiak-patent) explode at 130-140°C, no?
Imagine using boiling xylene(dimethylbenzene, SP ~140°C) instead.....more than likely....kawumm.....
[Edited on 12-2-2005 by BASF]
[Edited on 12-2-2005 by BASF]
|
|
|
thedestroyer5150
Harmless

Posts: 5
Registered: 12-2-2005
Location: Las Vegas
Member Is Offline
Mood: Eh..
|
|
Has anyone tried a synth with 100% HCL? That water content could be inhibiting DPPP production.
Human Life: Sexually transmitted disease; 100% fatal.
|
|
|
Joeychemist
Hazard to Others
  
Posts: 275
Registered: 16-9-2004
Location: Canada
Member Is Offline
Mood: Sedated
|
|
There is no such thing as 100% HCl, the highest I have seen is about 40%. 
|
|
|
$0meb0dy
Harmless

Posts: 13
Registered: 17-12-2003
Member Is Offline
Mood: No Mood
|
|
Maiby he means bubbeling HCl trough the aceton.
|
|
|
thedestroyer5150
Harmless

Posts: 5
Registered: 12-2-2005
Location: Las Vegas
Member Is Offline
Mood: Eh..
|
|
I mean concentrating it.
Human Life: Sexually transmitted disease; 100% fatal.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
You cannot have 100% liquid HCl, at least not at room temperature. Pure HCl is a gas, the common forms of HCl are aqueous solutions of it.
And if you want to concentrate it how would you do that? Boiling? Just remember if you attempt to concentrate HCl by boiling that gasses are less
soluble in water at higher temperatures. So boiling it would not work.
The only way possible that you could use HCl without water is to bubble the anhydrous gas into the acetone.
|
|
|
thedestroyer5150
Harmless

Posts: 5
Registered: 12-2-2005
Location: Las Vegas
Member Is Offline
Mood: Eh..
|
|
Cool gaseous compression like liquid oxygen. Or dry ice. But you're right, 100% can't really be done (for this purpose), but a higher
concenteation of about 60-80% can.
Human Life: Sexually transmitted disease; 100% fatal.
|
|
|
Atom
Harmless

Posts: 15
Registered: 1-1-2005
Member Is Offline
Mood: No Mood
|
|
Can't one concentrate HCl by freezing the water and pour the remaining liquid ( a higher percentage HCl ) in a flask?
Just like you can with H2O2
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
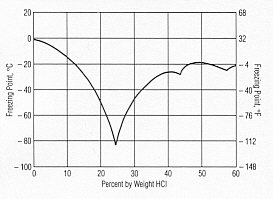
Freezing HCl
The attached graph may be of interest.
Meaning, concentrating HCl might be difficult this way.
edit: picture from http://www.resistoflex.com/hci_graphs.htm
sparky (^_^)
[Edited on 13-2-2005 by sparkgap]
|
|
|
Atom
Harmless

Posts: 15
Registered: 1-1-2005
Member Is Offline
Mood: No Mood
|
|
I cant see why it is difficult, 'cause water just freezes at 0C and you can get as much water out of your solution as wanted. Cant you?
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
| Quote: | | Originally posted by BASF Another thing: somebody mentioned using hot toluene as recrystallizing solvent.....isnt that hazardous? - toluene
boils at ~110°C....acetone peroxides (which are most likely major products when following the mackowiak-patent) explode at 130-140°C, no?
|
The toluene was heated in a sealed jar which was sitting in a hot water bath at 90 C .
I used a teflon rare earth stirbar and a solid teflon stirring rod for manual stirring sometimes when the sludge of crystals would solidify so much on
standing for a few minutes as to stall the stirbar in the mixture which was almost as stiff as wet sand . How safe it is to do this I don't
really know . It is not a mixture which I would subject to rough handling with glass on glass , but I supposed it would probably be okay with teflon
to glass .
And the temperature wasn't kept high for very long , just sufficiently to get everything in solution .
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
This was found by typing in "mackowiak" (inventor of patent) in scifinder.....
Note that this is the ONLY hit using the name of the inventor.
(acronym and also Beilstein Crossfire`s drawing function: no hits)
Bibliographic Information
Explosive diphoron pentaperoxide. Mackowiak, Wolfgang. Ger. Offen. (1971), 4 pp. CODEN: GWXXBX DE 1951660 19710422 Patent written in
German. Application: DE 69-1951660 19691014. CAN 74:143985 AN 1971:143985 CAPLUS
Patent Family Information
Patent No. Kind Date Application No. Date
DE 1951660 A 19710422 DE 1969-1951660 19691014
Priority Application
DE 1969-1951660 A 19691014
Abstract
The H2O-insol. title compd. (I) of 9000 m/sec detonation velocity and 200 explosion temp., useful for blastings in mines and quarries or for
military purposes, was prepd. with 90% yield from 1:1:2 Me2CO, HCl, and H2O2. I has the formula C18H26O2(O2)5.
Patent Classifications
IPC: C07D; C06B.
Indexing -- Section 50 (Propellants and Explosives)
Explosives
(diphorone pentaperoxide)
32619-19-5
Role: USES (Uses)
(explosives)
Registry Number: 32619-19-5
Formula: C18 H26 O12
CA Index Name: 1,2-Dioxolo[4,3-c][1,2]dioxepin-8(3H)-one, 6,6'-[dioxybis(methylene)]bis[tetrahydro-3a,6-dimethyl- (9CI)
Other Names: Diphorone pentaperoxide
-- Properties --
Calculated
Property Value Condition Note
Bioconc. Factor 17.1 pH 1 (1) ACD
Bioconc. Factor 17.1 pH 4 (1) ACD
Bioconc. Factor 17.1 pH 7 (1) ACD
Bioconc. Factor 17.1 pH 8 (1) ACD
Bioconc. Factor 17.1 pH 10 (1) ACD
Boiling Point 457.0±45.0 °C Press: 760.0 (1) ACD
Torr
Enthalpy of Vap. 71.71±3.0 kJ/mol (1) ACD
Flash Point 197.4±51.8 °C (1) ACD
Freely Rotatable Bonds 5 (1) ACD
H acceptors 12 (1) ACD
H donors 0 (1) ACD
Koc 265 pH 1 (1) ACD
Koc 265 pH 4 (1) ACD
Koc 265 pH 7 (1) ACD
Koc 265 pH 8 (1) ACD
Koc 265 pH 10 (1) ACD
logD 1.92 pH 1 (1) ACD
logD 1.92 pH 4 (1) ACD
logD 1.92 pH 7 (1) ACD
logD 1.92 pH 8 (1) ACD
logD 1.92 pH 10 (1) ACD
logP 1.924±0.967 (1) ACD
Molar Solubility Sparingly Soluble pH 1 (1) ACD
Molar Solubility Sparingly Soluble pH 4 (1) ACD
Molar Solubility Sparingly Soluble pH 7 (1) ACD
Molar Solubility Sparingly Soluble pH 8 (1) ACD
Molar Solubility Sparingly Soluble pH 10 (1) ACD
Molecular Weight 434.39 (1) ACD
Vapor Pressure 1.54E-8 Torr Temp: 25.0 °C (1) ACD
Notes:
(1) Calculated using Advanced Chemistry Development (ACD/Labs) Software Solaris V4.76 ( 1994-2005 ACD/Labs)
-- Resources --
References: ~1
STN Files: CAPLUS, CA
Deleted Registry Number(s): 11067-76-8
Database: REGISTRY
Additional info calculated with ACD-labs demo-version: density=1,3g/ccm (=> thus high Vdet very unlikely!!)
Note the low vapour-pressure. (=> thus rapid sublimation very unlikely!!)
My conclusion is that Wolfgang Mackowiak did eventually only file a patent without ever publishing additional scientific work on the subject ......
this is at least very strange...
[Edited on 14-2-2005 by BASF]
|
|
|
Geomancer
Hazard to Others
  
Posts: 228
Registered: 21-12-2003
Member Is Offline
Mood: No Mood
|
|
Personally, I think the patent is bunk. Just for fun though, let's advocate this devil.
First there is the illustration Mega found. If not traceable back to the original patent, it might mean the compound can be made, even if not by
the process in the patent.
Second, we know the first step (aldol to phorone) happens, under the right conditions. This is well documented. So, the (hydro)chlorination is the
sticking point.
But this is a free radical reaction right? That's why the H2O2 is labeled as a catalyst. It provides the source of the radicals that cascade
to chlorinate the thing. Note that one of the locations substituted is allylic, and so particularly susceptable to this kind of thing. And that leads
us to the third point: no one has taken steps to insure the generation of these radicals. I think that a catalyst for the decomposition of the
peroxide has been ommited from the patent. My knowledge of this type of chemistry is now expended. Perhaps Iron or Manganese will work?
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The peroxidation mechanism is highly unlikely
to occur in a manner decribed by the patent even if you get past the fact that the chlorinated precursor doesn't form as proposed by the patent
reactions . Usually when hydrogen peroxide reacts , it gives up only one oxygen , forming water as a byproduct , something of a "condensation
reaction" which adds the
"active oxygen" from the hydrogen peroxide to an already present oxygen residing on the organic compound , thereby forming an "organic
peroxide" .
However in the patents proposed reactions , the complete pair of oxygens is
split off from the two hydrogens of the hydrogen peroxide , and the pair of oxygens with peroxidic bond intact is then added to the chlorinated
organic compound , occupying the sites of chlorination preferentially to the chlorine which is split off and further reacts with the hydrogen pair
from the cleaved hydrogen peroxide to form two HCL's .
This entire scenario would be far fetched even for singlet ions to participate in such a reaction , and for such a reaction to proceed by involvement
of * PAIRS * of
hydrogens , oxygens , and chlorines is just preposterous on its face .
|
|
|
Joeychemist
Hazard to Others
  
Posts: 275
Registered: 16-9-2004
Location: Canada
Member Is Offline
Mood: Sedated
|
|
Maybe this compound could be more easily attained through use of a commercial ozone generator? If the patent was correct in preparing the right
precursor (probably not) than ozone could be a plausible route to venture, no? It could provide the intact -O-O- to take the place of the Cl moles,
but then again the patent would have to be totally right about the chlorinated precursor for that to work.
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by vulture
Painkilla made a good suggestion of summarizing this topic to get rid of all bloated speculation, but unfortunately I don't have much time on my
hands right now, so therefore I'm asking if there are a few members who'd like to condense this into a new thread. |
Just though that yes, I'm working on it  I started last weekend, and have
gone through 16 pages of this thread, finding the important pieces to be transferred to a summarized version, although all experimental observations
etc will remain intact, I'll just edit them to make them contain info from later posts and fix alot of punctuation and spelling mistakes. I started last weekend, and have
gone through 16 pages of this thread, finding the important pieces to be transferred to a summarized version, although all experimental observations
etc will remain intact, I'll just edit them to make them contain info from later posts and fix alot of punctuation and spelling mistakes.
There is a very large amount of very useful experimental data that will be VERY useful once it is transferred to a organized form without a large
amount of bickering, argueing and useless information (in this case) such as speculation on practical applications that fills a very large amount of
this thread's content.
Should I assemble this into a thread by posting several replies to a new topic summing up this thread or make a pdf out of it? It would be handy to
have a summarized thread on the forum in my opinion.
|
|
|
Geomancer
Hazard to Others
  
Posts: 228
Registered: 21-12-2003
Member Is Offline
Mood: No Mood
|
|
The peroxidation is plausible. I looked about in the library and it seems this kind of thing happens all the time. A large book entitled "Organic
Peroxides: Volume 1" specificly says that ROOR can be made from 2 RX with 1 H<sub>2</sub>O<sub>2</sub>. Conditions
aren't mentioned. March seems to say it happens in basic solution. Reagents for Organic Synthesis shows an intramolecular peroxide formation
knocking out Bromine, using H<sub>2</sub>O<sub>2</sub> and Silver Triflouroacetate. Many of these reactions proceed by ionic
means, though. If the peroxidation is ionic, I don't yet see why it doesn't add to the ketone as well. CuCl might be advantagious at some
point.
No, I will not give primary references. I don't have much time and my library isn't all that great for chemistry anyway.
|
|
|
| Pages:
1
..
31
32
33
34
35
..
37 |