DougTheMapper
Hazard to Others
  
Posts: 145
Registered: 20-7-2008
Location: Michigan, USA
Member Is Offline
Mood: Energetic
|
|
1,2-Dinitroguanidine and Salts
Apparently the nitration of guanidine continues past the mononitro- state as long as the mixed acid bath remains strictly anhydrous. In this book, (Klapötke, p. 162) it states that 1,2-dinitroguanidine is prepared by nitrating guanidine using 100% H2SO4 + SO3 in combination with
100% HNO3. This is also stated in what little of this article I was able to retrieve, stating that 100%H2SO4 should be used with 98% HNO3 with 2% N2O5 dissolved in.
The compound is then extracted from the nitration bath using ethyl acetate since 1,2-dinitroguanidine is reactive towards water.
The resulting product is nucleophilic and can form salts.
Axt has a beautiful prep of nitroguanidine here. The product of this reaction could then be subjected to the anhydrous nitration bath to form 1,2-dinitroguanidine which is then extracted
with EtOAc.
Ammonium Dinitroguanidine
Particularly interesting is ammonium dinitroguanidine as described in this paper on page 5, citing ammonium dinitroguanidine (ADNQ) having an EXPLO5 calculated detonation velocity of 9066m/s.
Looking into the synthesis of this compound, Klapötke states on p. 163 that ADNQ is synthesized from 1,2-dinitroguanidine and ammonium carbonate in
the presence of EtOH and water.
(Doing my best with the citations. How should I reference these?)
-DTM
Victor Grignard is a methylated spirit.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
from here _
http://www.sciencemadness.org/talk/viewthread.php?tid=14033#...
Recently isolated Dinitroguanidine and it's derivative Dinitrobiguanide show acidic
character and also form salts as do the other Nitramine compounds.
See article page 509 , pdf pg 9 _ here
Synthesis and Some Properties of 1,2-Dinitroguanidine http://ifile.it/0gzni9v
or here _ http://www.sciencemadness.org/talk/files.php?pid=187259&...
Attachment: Biguanide.pdf (99kB)
.
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Quote: Originally posted by DougTheMapper  |
Looking into the synthesis of this compound, Klapötke states on p. 163 that ADNQ is synthesized from 1,2-dinitroguanidine and ammonium carbonate in
the presence of EtOH and water.
|
Water? Really? You said, that DNG is reactive towards water 
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Dinitroguanidine
Dinitroguanidine is the product of nitration of nitroguanidine. The reaction rate is much faster with a 2% solution of N2O5 in HNO3. Dinitroguanidine
dissolved in water solution, slowly hydrolyzes after prolonged standing to precipitate nitroguanidine. It is moderately soluble in both water and
organic solvents and the solubility quickly increases on warming. At 20C its solubility in water is 53g/L, in ethyl acetate 86g/L.
Dinitroguanidine can be recrystallized form alcohol or acetic acid, being a sufficiently stable compound with a melting point of 169C. Nitroguanidine
forms salts with alkali dissolved in alcohal. Under sufficient acidic conditions, Dinitroguanidine hydrolyzes to nitroguanidine, while under only
moderately acidic conditions nitrourea is formed.
Russian Journal of Organic Chem, Vol39, #4, p501
Synthesis and some Properties of 1,2-Dintroguanidine
A.A.Astrat’yev, D.V. Dashko
State Technological Institute,
St. Petersburg, Russia
Ammonium dinitroguanidine (the dinitroguanidine salt of ammonia) decomposes at 197 °C, and has an impact sensitivity greater than 10 Joules, and
friction sensitivity greater than 252 Newtons. The calculated detonation velocity of this salt is 9066 m/sec, with calculated detonation pressure of
332 kbar, The detonation velocity was experimentally measured and is consistent with the calculated value. From these values, ammonium
dinitroguanidine shows greater explosive performance than HMX.
Yes, dinitroguanidine will very slowly react when dissolved in water. Nitrourea also slowly hydrolyses with water.
[Edited on 1-11-2011 by AndersHoveland]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AndersHoveland  | " ammonium dinitroguanidine shows greater explosive performance than HMX "
" calculated detonation velocity of this salt is 9066 m/sec, with calculated detonation pressure of 332 kbar " |
pressure is comparable to RDX , 340 kbar , HMX is 9100 m/s , 390 kbar
I'm not nit picking , but inaccurate assertions spread disinformation contagion if not corrected.
.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Agreed, I was just quoting the literature. Obviously their comparison was inaccurate. Although the detonation velocity for the dinitroguanidine salt
of ammonia is higher than that for RDX. (8750 m/sec for RDX)
Read somewhere in the literature that dinitroguanidine has nearly the same sensitivity (resistance to impact) as dinitrourea, with similar explosive
performance also.
One wonders if a dinitroguanidine, or dinitrourea, salt of hydrazine is possible, assuming the dinitroguanidine is not oxidizing enough to react with
the hydrazine.
[Edited on 2-11-2011 by AndersHoveland]
|
|
|
DougTheMapper
Hazard to Others
  
Posts: 145
Registered: 20-7-2008
Location: Michigan, USA
Member Is Offline
Mood: Energetic
|
|
Here's a related one, just in case those aren't powerful enough for ya:
3-amino-1-nitroguanidine nitrate (ANGN) from that paper again.
RDX.......................340kbar 8750m/s @ 1.82g/cc
HMX.......................390kbar 9100m/s @ 1.91g/cc
ANGN.....................419kbar 9750m/s @ 1.905g/cc
Margin over HMX +7.4% +7.1% -0.3%
I can't find anything about a synthesis. Aminoguanidine can be synthesized from nitroguanidine and zinc acetate, as seen here, albeit at a reported >50% yield.
I'm not too educated about the nitration of amines but I believe amines need to be protected or you'll end up with tar. But aminoguanidine only has 1
carbon atom and it's already surrounded with amine-like structures, so....? Anyway, it would be nice if aminoguanidine could just be straight-up
nitrated. I don't see why not.
[Edited on 3-11-2011 by DougTheMapper]
Victor Grignard is a methylated spirit.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Nitroguanidine can react with one equivalent of hydrazine hydrate to form nitroaminoguanidine,
CN3H4NO2 + N2H4 --> NH2NHC(=NH)NHNO2 + NH3
Nitrate Salt of Nitroguanidine
When nitroguanidine is dissolved in hot, concentrated nitric acid and allowed to crystallize, the nitrate salt is deposited in thick rhombic shaped
prisms m.p. 147ºC , with decomposition. It loses nitric acid slowly in the air and yields α-Nitroguanidine when recrystallized from water.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Wow. Just guanidine nitrate, H2SO4, azeotropic HNO3, and hydrazine required to make something more powerful than HMX, all without a nitration using
concentrated HNO3. With sensitivity similar to RDX, and neutral OB too. I can accept that it's stable only up to 130*C with those numbers. A mixture
with some of the free base (VOD 8977) might improve the stability?
This almost makes tetrazoles look not worth it, at least as the secondary.
Edit:
There is lots of nice sensitivity data in that reference Doug linked to.
So using engager's guanidine nitrate synthesis, you could theoretically make about 200-220g of the aminonitroguanidine nitrate from 320g urea, 340g
NH4NO3, 500g conc H2SO4, and about 180g hydrazine sulfate. Looks like fun.
[Edited on 4-11-2011 by 497]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I do not know, but one would think the sensitivity would not be any greater than hydrazinium nitrate, which is near that of RDX.
|
|
|
DougTheMapper
Hazard to Others
  
Posts: 145
Registered: 20-7-2008
Location: Michigan, USA
Member Is Offline
Mood: Energetic
|
|
ANGN seems to be at least equally, if not less, sensitive than RDX.
ANGN
Impact Sens. 10 J
Friction Sens. 120 N
Electrostatic 0.5 J
Decomp. T 130 C
RDX
Impact Sens. 7 J
Friction Sens. 120 N
Electrostatic 0.1 - 0.2 J
Decomp. T 210 C
And since a blend of ANG/ANGN was suggested to increase stability:
ANG
Impact Sens. 20 J
Friction Sens. 144 N
Electrostatic 0.15 J
Decomp. T 184 C
But in all seriousness, you can fire a bullet through RDX-based C4 without it going off. ANGN is even less impact sensitive than that. Its low thermal
stability and slow reactivity with water are a bit of a safety feature: it is likely destroyed through hydrolysis by hot water/steam. Besides, if
your energetics are seeing 130C, you have issues anyway.
Military? Probably not.
The holy grail of amateur energetics? Quite possibly.
All we need to do now is design an efficient synthesis, attempt, and characterize. I have access to NMR spectroscopy where I go to school. Maybe I'll
bring this up with the department chair of chemistry where I go to school (we're good friends) and I can attempt the synthesis somewhere other than
the dingy workbench in my basement.
(The guy is a huge supporter of amateur chemistry. When I first met him, he had a big jar of nitrocellulose on his desk for a demo he was doing. I may
have turned him on to this website as well.)
[Edited on 4-11-2011 by DougTheMapper]
Victor Grignard is a methylated spirit.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Could a compound similar to ANGN be made from urea rather than guanidine? I know that hydrazine reacts with urea to form aminourea. It would be very
convenient if 1-amino,3-nitro-urea be obtained by reacting nitrourea with hydrazine. Unfortunately, I have some doubts about this because nitrourea
gradually hydrolyzes with water into cyanic acid and nitroamine [which itself breaks down in the water, giving off nitrous oxide]. Perhaps anhydrous
hydrazine would need to be used. Urea pellets are readily available.
Sodium and zinc salts of nitrourea exist, so nitrourea does not necessarily always hydrolyse in alkaline solution.
[Edited on 15-11-2011 by AndersHoveland]
|
|
|
DougTheMapper
Hazard to Others
  
Posts: 145
Registered: 20-7-2008
Location: Michigan, USA
Member Is Offline
Mood: Energetic
|
|
<b>Ok, so here's my practical synthesis of ANGN:</b>
First, a note:
Before I try this, I need some info. I would also appreciate any feedback regarding this proposed synthesis.
List of Reagents:
Conc. H2SO4
NH4NO3
Urea
Hydrazine sulfate
NaOH
Conc. HNO3
<b>1. Synthesize guanidine nitrate</b>
Melt urea with NH4NO3 @ 190C with stirring. Dissolve, filter, crystallize. Yield: Guanidine nitrate @ 65-70% theo.
http://www.sciencemadness.org/talk/viewthread.php?tid=14342 (Engager, "Chapter 2 – Aminoguanidine, nitroguanidine route")
<b>2. Convert guanidine nitrate to nitroguanidine.</b>
Heat guanidine nitrate in conc. H2SO4. Filter, wash. Quantitative synth, Orgsyn reports 85-90% yield from solubility loss during washing.
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv1...
See also:
Engager, "Chapter 2 – Aminoguanidine, nitroguanidine route"
<b>3. Convert nitroguanidine to aminonitroguanidine </b>
"Nitroguanidine can react with one equivalent of hydrazine hydrate to form nitroaminoguanidine,
CN3H4NO2 + N2H4 --> NH2NHC(=NH)NHNO2 + NH3" (AndersHoveland, this thread)
I found this claim on slide 10 of this presentation but it reveals almost nothing other than that this reaction takes place in the presence of water.
Cited on that page is the reference "J. A. Castillo-Mendelez, B. T. Golding, Synthesis 2004, 10, 1655-1663." I have been so far unable to find a copy
of this book or the pages mentioned. Can anyone help with that?
<i>Anders, do you have any documentation behind this? Any details at all about a synth would be great!</i>
<b>4. Convert aminonitroguanidine to its nitrate salt</b>
"Nitrate Salt of Nitroguanidine
When nitroguanidine is dissolved in hot, concentrated nitric acid and allowed to crystallize, the nitrate salt is deposited in thick rhombic shaped
prisms m.p. 147ºC , with decomposition. It loses nitric acid slowly in the air and yields α-Nitroguanidine when recrystallized from water."
(AndersHoveland, this thread)
We can infer that if nitroguanidine + HNO3 --> nitroguanidine nitrate, then ANG +HNO3 --> ANGN in a similar fashion.
Thoughts?
-DTM
[Edited on 16-11-2011 by DougTheMapper]
Victor Grignard is a methylated spirit.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Obviously dilute nitric acid. If it is concentrated to any extent it could oxidize the hydrazine. ANG is a much better base than
nitroguanidine, so ANGN would be much more "stable" (in terms of the acid being neutralized) than nitroguanidine nitrate.
"Diazotization of nitroaminoguanidine"
T. E. O'Connor, G. Fleming, J. Reilly
"NITRO-AMINOGUANIDINE"
Ross Phillips, John F. Williams
J. Am. Chem. Soc., 1928, 50 (9), pp 2465–2470
| Quote: |
Preparation of Nitro-aminoguanidine
Hydrazine suldare, 32,53 g, was placed in a 2000-cc. flask with 200 cc. of distilled water and 500 c.c. of N ammonia water. When the hydrazine sulfate
had dissolved, 26 g. of nitroguanidine was added. The flask was then heated to 50-60degC, during which time the nitroguanidine went into solution,
nitrous oxide gas began to evolve and the reaction liquor turned to an orange-red color. In about one houe at the above temperature, gas ceased to
form and the liquor was rapdily evaporated to about one third of its volume. On cooling, a white crystalline powder separated which was filtered off,
washed with cold water and dried in the air; yield 13.1 g.
|
http://pubs.acs.org/doi/abs/10.1021/ja01396a020
But I am sure the reaction between urea and hydrazine is essentially no different than the reaction between ammonium nitrate and hydrazine,
essentially ammonia is released.
[Edited on 18-11-2011 by AndersHoveland]
|
|
|
Motherload
Hazard to Others
  
Posts: 245
Registered: 12-8-2012
Location: Sewer
Member Is Offline
Mood: Shitty
|
|
I couldn't find any reference on this but I was curious about replacing Di Nitro Urea with Di Nitro Guanidine in the synthesis of Keto RDX.
In hopes to replace the >C=O with a >C=N-H which might further oxidize into a
>C=N-NO2
Any thoughts ?
"Chance favours the prepared mind"
"Fuck It !! We'll do it live !!"
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Motherload  | I couldn't find any reference on this but I was curious about replacing Di Nitro Urea with Di Nitro Guanidine in the synthesis of Keto RDX.
In hopes to replace the >C=O with a >C=N-H which might further oxidize into a
>C=N-NO2
Any thoughts ? |
I think there could still only be two nitramine groups on the guanidine moiety, but with one of the nitro groups on the amine group outside
the ring. The resulting explosive would still only have 3 nitramine groups per molecule. Furthermore, you might want to minimize the proportion of
formaldehyde in the reaction, to prevent cross-bridging of two molecules (the extra -CH2- would not help OB)
[Edited on 17-3-2013 by AndersHoveland]
|
|
|
Motherload
Hazard to Others
  
Posts: 245
Registered: 12-8-2012
Location: Sewer
Member Is Offline
Mood: Shitty
|
|
I am not condensing it with formaldehyde.
In a mix of WFNA and Oleum Nitroguanidine is added to make Dinitroguanidine DNG..
To this mix .... Hexaminedinitrate HDN is added.
This should form a Keto-RDX like structure with three >N-NO2
And hopefully along with a >C=N-NO2 at position one.
That's my proposition.
"Chance favours the prepared mind"
"Fuck It !! We'll do it live !!"
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Motherload  | I am not condensing it with formaldehyde.
In a mix of WFNA and Oleum Nitroguanidine is added to make Dinitroguanidine DNG..
To this mix .... Hexaminedinitrate HDN is added.
This should form a Keto-RDX like structure with three >N-NO2
And hopefully along with a >C=N-NO2 at position one.
That's my proposition. |
I think you would just get this:
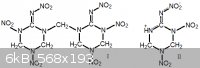
One of the groups would be a nitramino group, rather than a regular nitramine. Since the amine group would become electron donating to the
nitramino group, its chemistry is considerably altered.
Hexamine has a higher equivalent ratio of formaldehyde than ammonia, so you would probably get a bridged dimer, shown in figure (I). It would not get
futher nitrated because those nitrogen centers are electron donating.
if you look at the correct structure of dinitroguanidine, it contains a nitramino group: http://www.chemspider.com/Chemical-Structure.10216019.html
(you might also see at the structure of 5-nitraminotetrazole)
[Edited on 19-3-2013 by AndersHoveland]
|
|
|
Motherload
Hazard to Others
  
Posts: 245
Registered: 12-8-2012
Location: Sewer
Member Is Offline
Mood: Shitty
|
|
Why do you suspect a NH+ in compound 2 vs a N-NO2 ?
"Chance favours the prepared mind"
"Fuck It !! We'll do it live !!"
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
It is a little complicated to explain. The only way to explain it is to show a resonance diagram. In fact, let me draw one for dinitroguanidine.
The structure of dinitroguanidine would typically be drawn as:
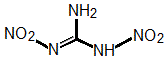
But actually the bonding in the entire molecule is delocalized. There are no "real" double bonds. Both nitro groups are electron withdrawing.
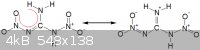
(for obvious reasons, I am not going to draw all the possible resonance diagrams out, but hopefully you get the idea)
The only difference between (I) and (II) was the -CH2- bridge.
The NH+ was not a stationary positive charge, but rather a delocalized one. It would be a very polar molecule.
[Edited on 19-3-2013 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I did find this:
| Quote: |
1-Amino-3-nitroguanidine (ANQ, 1 ) was synthesized by hydrazinolysis of nitroguanidine (NQ) with hydrazine hydrate.
1-Amino-3-nitroguanidine (ANQ) can be described as the aminated sister compound of the famous 1-nitroguanidine (NQ). The next step in the preparation
of mixed aminonitroguanidines would be the synthesis of 1,3-diamino-5-nitroguanidine (DANQ), which is completely unknown yet.
Interestingly, only very little correspondence on aminonitroguanidine is found in the literature.
We recently investigated the use of ANQ in energetic materials in its neutral as well as protonated form. For example, highly energetic
1-amino-3-nitroguanidinium dinitramide has been described. Because dinitraminic acid (HN(NO2)2) and concentrated aqueous solutions can explode
spontaneously, the reaction pathway using a metathesis reaction of amino-nitroguanidinium chloride with silver dinitramide was chosen. This example
shows the utility of the herein described compounds as precursor materials for the synthesis of ionic energetic materials containing the
1-amino-3-nitro-guanidinium cation when using metathesis reaction protocols to precipitate low soluble silver halides in case of reacting the ANQ
halides with the corresponding silver salts of the used acids or to precipitate BaSO4> in case the corresponding Ba-salts are reacted with the
herein described sulfate salt of ANQ. Here we present the synthesis and characterization of three amino-nitroguanidinium halides and
bis(amino-nitroguanidinium) sulfate.
Synthesis
The synthesis of 1-amino-3-nitroguanidine ( ANQ) was achieved starting from commercially available nitroguanidine (NQ) by treatment with hydrazine
hydrate. In a hydrazinolysis reaction, the aminated nitroguanidine is formed after the elimination of ammonia. Unprotonated ANQ shows rather poor
solubility in water, so that it can be isolated from the reaction mixture after neutralization by suction filtration. Unlike in water or buffered
neutral solutions, in acidic media ANQ is dissolved comparatively easily upon the formation of the protonated ANQ species. Therefore, the halides as
well as the sulfate salt can be prepared by dissolving ANQ in dilute aqueous solutions of the respective mineral acids HCl, HBr, HI and H2SO4. To
fully synthesize and characterize all halides (F−, Cl−, Br−, I−) of ANQ, it was
also dissolved in 40% aqueous HF. Unfortunately it was only possible to isolate unprotonated ANQ from this reaction mixture. The reason for this
behavior is easily found in the pK values of the used mineral acids. ANQ, due to the presence of the electron withdrawing character of the nitro
group, is a comparatively weak base and requires strong mineral acids for protonation in aqueous media. Since the acidic strength of the hydrohalides
in aqueous solution decreases in the order HI > HBr > HCl > HF, the latter one is not able to protonate ANQ in aqueous solution any more.
However, 40% aqueous HF proved to be an excellent solvent for the recrystallization of the poorly water soluble ANQ, especially if single crystals,
e.g., for single crystal X-ray diffraction, are needed. The storage stability of the halides decreases with increasing molecular weight of the anion.
Whereas the hydrochloride remains a colorless crystalline material even after several months, the hydrobromide discolors slightly and the hydroiodide
turns completely dark after the release of I2 indicating a decomposition of the material.
Sensitivity Testing
Since 1-amino-3-nitroguanidinium salts show enhanced sensitivity towards outer stimuli such as impact and friction, the impact and friction
sensitivities were determined and carried out...
Regarding the sensitivities of the salts, a large difference between the halides and the sulfate salt is also observed. Whereas the halides reveal
sensitivities of 25 J (impact sensitivity) and 288 N (friction sensitivity), the sulfate salt is much more sensitive, having 6 J (impact sensitivity)
and 120 N (friction sensitivity). This can primarily be explained by the formation of monohydrates, which is true for all halides, whereas the sulfate
salt crystallizes water-free. The determined values imply a classification of the tested materials as “sensitive” towards both impact and
friction. The higher sensitivities of the protonated species discussed herein compared to the neutral compound ANQ can be correlated to a lowered
C–N bond order of the hydrazine moiety of the molecule, which appears upon protonation of the molecule as it was found during the structural
investigation of neutral ANQ and its protonated species in reference 4. The same argumentation can also be applied to the thermal stabilities of the
compounds, whereas a weaker C–N bond of the hydrazine moiety facilitates the loss of the hydrazine moiety and thus, decomposition of the material.
"Inorganic Amino-Nitro-Guanidinium Derivatives", Niko Fischer, Thomas Klapötke, Karin Lux, Franz Martin, Jörg Stierstorfer, University of
Munich, (2012)
|
|
|
|
malford
Hazard to Others
  
Posts: 116
Registered: 17-6-2013
Member Is Offline
Mood: No Mood
|
|
So, has anyone here synthesized ammonium dinitroguanidine? It has caught my attention.
|
|
|
MineMan
National Hazard
   
Posts: 996
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Anybody have any luck with any of these compounds??
|
|
|
Mr.Greeenix
Harmless

Posts: 40
Registered: 8-4-2015
Member Is Offline
Mood: No Mood
|
|
This fits in here perfectly. 
Attachment: 1-Amino-3-nitroguanidine (ANQ).pdf (3.9MB)
This file has been downloaded 773 times
|
|
|