| Pages:
1
2 |
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
1,2-dinitro cyclopropane and nitric acid?
I've been thinking about a mono-propellent of 1,2-dinitro cyclopropane and nitric acid, passed through a bed of pyrosulfuric acid imbedded beads? The
reaction of the nitro-cyclopropane with the nitric acid to produce 3-6 nitro groups should create enough heat to ignite the highly sensitive
trinitrocyclopropane and others. The main problem I can think of is the pyrosulfuric acid would quickly be vaporized and then..well the catalyst
would be gone. Also I would need to maintain a very chilly temperature on the tube just before entering the chamber to prevent ignition or detonation
traveling up the tube, liquid helium?.
Any-thoughts guys?
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Why so exotic? A bed of some..acid? Cyclopropane? Premixed with other acid? I thought the monopropellant concept is to be simple?
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
Quote: Originally posted by Ral123  | | Why so exotic? A bed of some..acid? Cyclopropane? Premixed with other acid? I thought the monopropellant concept is to be simple?
|
Its more to reduce weight of equipment, one fuel/oxidizer tank instead of two weighs one tank, and even less than three. Dia(?)fuels are pretty
simple--mono-propulsion is a little more complex, ex. hydrogen peroxide rockets pass 90+% hydrogen peroxide through a bed of catalyst (usually
platinum-silver screen) and Otto fuel II is propylene glycol dinitrate (the major component), 2-nitrodiphenylamine, and dibutyl
sebacate[http://www.atsdr.cdc.gov/tfacts77.html] which is far more complicated then many diapropelents. The exception is generally when it comes to
solid fuel, which can be as simple as black-powder in a tube with a nozzle--but the problem with solid fuel is it doesn't carry enough energy per gram
to get anything (deep) into space with (relative ease). There's a reason NASA uses H2 O2 rockets, but the problem with H2 O2 is that it needs alot of
equipment to keep it functional (and stable) so small rockets cannot feasibly be sent to space using oxyhydrogen as a fuel/oxidizer. Other fuels like
hydrazine could solve that problem, but they're unstable and moderately expensive so once again they're not favored for small rockets. Currently
rockets that could take up much heavier equipment ave being used to send up lighter stuff--this is fiscally inefficient. My goal is to design a
monopropelent rocket that could be altered and scaled up to send small probes to other planets (either landers or orbiters) that is less expensive
then the conventional high specific impulse diapropelents, and with a higher specific impulse then conventional low specific impulse monopropelent
rockets. Trinitrocyclopropane caries alot of energy because those bent bonds on the cyclopropane hold alot of energy, but are also weaker then a
regular single bond because of increased strain. I'm also looking at stabilizing additives of more stable cycloalkanes, cycloalkenes, and
cycloalkynes. Such as Dicyclobutane, and 1-methylcyclobutane, and benzene as well as 2-nitrodiphenylamine, and dibutyl sebacate from Otto fuel II.
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Tetranitromethane with toluene would give good det vel. ooops impulse I ment  Why monopropellant with impulse anywhere near cryogenic O2/H2 wouldnt be extremely totaly detonateable? Solid fuels arent so bad as impulse. If a
liquid system doesnt use turbopomps and the engine is pressure fed there wouldnt be significant advantage over solid fuels. So cheap stuff in to
space... I think for anything serious a two component fuel is needed. At least dioxide/kerosine or better. The rest I think is more about efficient
mass production technology.
Why monopropellant with impulse anywhere near cryogenic O2/H2 wouldnt be extremely totaly detonateable? Solid fuels arent so bad as impulse. If a
liquid system doesnt use turbopomps and the engine is pressure fed there wouldnt be significant advantage over solid fuels. So cheap stuff in to
space... I think for anything serious a two component fuel is needed. At least dioxide/kerosine or better. The rest I think is more about efficient
mass production technology.
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
If I know what your saying (you've got some grammar issues)... The idea is stabilized 1,2-dinitro cyclopropane and nitric acid supercooled would not
detonate, if its above its flashpoint at all. But the 1,2,x-trinitrocyclopropane and higher species (produced in the combustion chamber) would
detonate producing a high specific impulse fuel on demand. You might be right anyways though; its not going to be a failure if I can't get it
feasible for space travel, it'll be cool if I can get it a mile up or more.--maybe I could use it to blow-up a cloud? jk
[Edited on 1-9-2012 by AirCowPeaCock]
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
I'm telling with good enough impulse rocket blows up surely(and you go to jail for explosive compounds). Monopropellants are not for main engine on
space rocket.
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
Quote: Originally posted by Ral123  | | I'm telling with good enough impulse rocket blows up surely(and you go to jail for explosive compounds). Monopropellants are not for main engine on
space rocket. |
Not yet
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
sorry for being noob and being certain at things at the same time. And also being rude over it 
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
Your not really being rude, your just saying the expected, a highly sensitive explosophore might blow an engine to bits. And I think because the
primary fuel would be produced sub-instantaneously--it wouldn't. I could be wrong though, I can't find much information on 1,2-dinitro cyclopropane,
It might be to sensitive already, I might need to settle for mononitro cyclopropane..but that would probably ruin the whole plan...
[Edited on 1-9-2012 by AirCowPeaCock]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Just my opinion of your idea-
I am not completely sure, but 1,2-dinitro cyclopropane is probably a sensitive explosive. Even plain cyclopropane itself is unstable and can
spontaneously detonate if the diameter of the pipe it flows through is too large! Paradoxically, sometimes adding nitro groups can actually reduce the
sensitivity of certain sensitive explosive molecules, an example of this is diazo-nitroaniline perchlorate.
| Quote: |
You would thinking that more nitro groups would make the diazo perchlorate compounds more sensitive, but actually the reverse of this seems to be the
case! This seemingly paradoxical behavior can be explained by the additional intermolecular attraction between molecules from the pressence of the
nitro groups, the nitro groups can also be seen as further "diluting" the unstable diazonium group.
C6H5–N[+]ΞN ClO4[-]
Benzenediazonium perchlorate spontaneously detonates when left to dry under sunlight. It detonates when lightly tapped with a metal spoon, showing
very high sensitivity.
NO2C6H4–N[+]ΞN
Minute quantities of 3-nitrobenzenediazoperchlorate easily detonate on exposure to flame, without need for any confinement. The white substance
darkens in sunlight, first turning a medium brown, then dark brownish orange. There is no darkening if stored in the dark for several months. It does
not appear to be hygroscopic, but the salt cakes up.
(NO2)2C6H3–N[+]ΞN
The diazotization step for preparing 3,5-dinitrobenzenediazoperchlorate requires 60% concentrated sulfuric acid, as dilute nitrous acid by itself is
not sufficient to diazotize the amino group when there are two nitro groups on the benzene ring. Small quantities of
3,5-dinitrobenzenediazoperchlorate deflagrate when burned, whereas similar such quantities of 3-nitrobenzenediazoperchlorate would detonate.
|
You might alternatively consider cyclobutane instead, which is much more stable. 1,1-dinitrocyclobutane is probably the least expensive nitro
derivitive, as it can be prepared from cyclobutanone (oxime condensation, then NO2).
The pyrosulfuric acid would be unnecessary. Concentrated nitric acid would probably oxidize cyclobutane on contact, and certainly cyclopropane.
|
|
|
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
| Quote: | | Tetranitromethane with toluene. |
Skimming through this k3wlish thread I see some super shock-sensitive mixtures as proposed 'monopropellants'!
The liquid mixture above, has power exceeding that of HMX and the sensitivity of NCl<sub>3</sub>!
Darwin award, anyone?
P
|
|
|
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
| Quote: |
2.6 Synthesis of Compound 6: trans-1.2-Dinitrocyclopropane (DNCP).
Wade and co-workers' procedure<sup>9</sup> for the synthesis of trans-1,2-dinitrocyclopropane
(compound 6) was substantially improved for scale-up purposes. The
original and the improved procedures employ 1,3-dinitropropane in DMSO solution.
The original procedure used dimethylsodium as the base, and reaction temperature
and time were crucial. The original procedure is strongly exothermic and would not be
readily amenable to scale up.
11
The improved procedure involves treatment with 10 molar equivalents of
sodium acetate* as a mild base and 3 molar equivalents of iodine. Ring closure takes
place in approximately 2 hr. In our best run on a 100 mg scale, a 43% crude yield of
compound 6, which could be readily recrystallized to yield pure compound 6 (not
optimized, small scale), was obtained.
When scaled up, the improved procedure gave pure product by
recrystallization but provided a lower yield. A solution of 1,3-dinitropropane (2.0 g,
14.9 mmol) in DMSO (50 mL) was added dropwise over 5 min to a cooled solution
containing sodium acetate (12.3 g, 149 mmol) and iodine (11.4 g, 45 mmol) in DMSO
(200 mL). Cooling during the addition was performed so that the temperature did not
rise above 30 °C. In one run where the reaction temperature was not controlled and
rose to near 50 °C, a side product tentatively identified as 5-nitroisoxazole was formed
in significant quantity. The resulting solution was poured into ice-cold brine (2 L).
Extraction with ethoxylacetate (three 100-mL portions) followed by washing with brine
(three 100-mL portions), drying over anhydrous Na2S04, and concentration gave 1.86 g
of crude product. The product was recrystallized from CCl4-hexanes to give 223 mg of
pure compound 6 and a second crop of 83 mg of fairly pure compound 6. Chromatography
of the mother liquor provided more product, which was combined with the
second crop and recrystallized to yield 200 mg of pure compound 6 (423 mg total, 21%
yield). The procedure was repeated two times to prepare a total of 1.2 g of com-'
pound 6.
3. DETONATION EXPERIMENTS
Detonation studies were conducted on compounds 1-6 to determine which
compounds would be best to use in follow-through devices. Most of the detonation
studies involved using 25 mL of a nitromethane solution of the compound being tested
and employing morpholine as a sensitizer. The solution was placed in a sample tube
mounted on a plastic block (blast observation device). Polypropylene and high-density
polyethylene blocks were used.
The sample tube was fitted at the top with a blast cap and explosive
booster pellet placed touching the solution, and the device was detonated remotely
(Figure 1). The observation block typically developed a crater from the ensuing blast.
The size of the crater was measured by filling it with water and determining the volume
of water. The results obtained are presented in Tables 2-6. A number of experiments
involving detonation of sensitized nitromethane, not admixed with test compounds,
were performed to develop reference standards. The results of the reference
detonations are listed in Table 7. |
Have some real k3wl fun!!!
P
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
Awesome! Ill do that when I replace my fume hood windows with polycarbonate. I'm still going to look into a mixture that won't detonate based on
nitrocyclo alkanes, alkenes, and/or alkynes.
|
|
|
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
| Quote: | | Awesome! Ill do that when I replace my fume hood windows with polycarbonate. |
How's about going the whole hog and moving residence --- to a nuclear-underground-detonation-proof bunker?
And with all that insulation, your heating bills would be, er, quite reasonable, wouldn't you say? 
P
|
|
|
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
Well . . . He's only thirteen, after all ─ which is even younger than you, probably! 
P
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Better thirteen the then thirtyone  Shouldn't you old guys be moving important
businesses instead of mixing *** and watch what will happen? Shouldn't you old guys be moving important
businesses instead of mixing *** and watch what will happen?
|
|
|
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
Since when is 63 old?
Right now, I'm looking forward to 69!
If 'things' go to plan, that is? ( )( )( )( )( ) )
P
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
your brains are gushing from your eaaarrrrrrrrrrssss! so I wouldn't count on making it to 69 if I were you
|
|
|
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
Different frequencies, AirCowPeeCock?
The night isn't isn't over yet . . . and we both just happen to be night-owls!
But fuck her! She's late ─ again!
P
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Pulverulescent  |
2.6 Synthesis of Compound 6: trans-1.2-Dinitrocyclopropane (DNCP).
Wade and co-workers' procedure<sup>9</sup> for the synthesis of trans-1,2-dinitrocyclopropane |
It would be interesting to investigate whether that procedure could also be used to prepare 1,3-dinitrocyclobutane, using
1,3-dinitro,4-iodobutane as the precursor. 43% yield is very good for such a direct reaction considering the poor net yields for other typical routes
with many more steps.
Once prepared, 1,3-dinitrocyclobutane could easily be converted to the very difficult to make high performance explosive TNAZ. This compound is
actually mentioned as one of the experimental precursors in the literature.
[Edited on 12-1-2012 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
sorry, correction: I meant that 1,3-dinitrocyclobutane could easily be converted to
1,1,4,4-tetranitrocyclobutane, which is similar to the high performance explosive TNAZ.
For oxidizing a lone nitro into a geminal pair of nitro groups (two nitro's on the same carbon atom), the following may be useful.
An alcohol of an azetidine derivitive (which is a square ring) can be oxidized to the ketone ( =O ) with chromium trioxide. Reaction of this ketone
with hydroxylamine hydrochloride and aqueous sodium acetate (which served as a mild base) to form the oxime ( =NOH ). The oxime of this compound is
then treated with 99% HNO3 in methylene chloride, leading to oxidative nitration, in 40-50% yield with formation of germinal nitro groups [meaning two
nitro's on the same carbon atom].
“Synthesis of 1,3,3-Trinitroazetidine”, T. Axenrod, C. Watnick, H. Yazdekhasti, P.R. Dave, (1993)
So essentially cyclobutanol, C4H7OH, could be converted into 1,1-dinitrocyclobutane, C4H7(NO2)2
Also to mention that usually two nitro groups on either the same carbon atom, or two ajacent carbon atoms, are not thermally stable [there are several
exceptions that are too complicated for me to explain]. Fortunately in the case of highly strained rings [triangular or square], this lends thermal
stability to such nitro groups. By "not being thermally stable", it means that, for example, dinitroethane [either isomer] gradually decomposes at
ambient temperatures, but especially rapidly when warmed.
[Edited on 17-1-2012 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
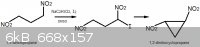
I would like to discuss the basic chemistry related to the
"Synthesis of Compound 6: trans-1.2-Dinitrocyclopropane" that was mentioned in this thread.
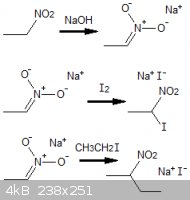
References
Journal of the Chemical Society, Volume 68, Part 1, p3
(note that the sodium salt of nitroethane typically is prepared in sodium hydroxide dissolved in pure alcohol. It undergoes more complex/degredation
reactions in the presence of water)
| Quote: |
nitroethane to its sodium salt and chlorination of the salt to give ... 93.8% chloronitroethane |
"Synthesis of 2,2-dinitropropanol, Studies on Continuous Preparation", Edward E. Hamel, AeroJet General Corporation, California
(1,1-dichloronitroethane will tend to form, but the mono-chlorinated compound can be formed using an excess of nitroethane)
"Chlorination of Nitromethane" US Patent 3096378
| Quote: |
nitroalkanes produces the corresponding nitronate anions which can be used as carbon nucleophiles in reactions with haloalkanes
|
"Nitroalkanes as key building blocks for the synthesis of heterocyclic derivitives", Roberto Ballini, Italy,
5th Issue, Eurasian Conference on Heterocyclic Chemistry
(for electrophilic substitution, iodine and bromine atoms will both substitute off readily, but the reaction rate for chlorine is negligable,
essentially inert, under ordinary conditions)
[Edited on 19-1-2012 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
trans-1,2-dinitrocyclopropane has a density of 1.59 g/cm3.
("trans-" meaning the nitro groups are sticking out towards opposite planar sides of the triangular ring)
Wade, same reference as above
Quote: Originally posted by AndersHoveland  |
(note that the sodium salt of nitroethane typically is prepared in sodium hydroxide dissolved in pure alcohol. It undergoes more complex/degredation
reactions in the presence of water)
|
For information about the reaction of the sodium salt of nitromethane in water, you can see the threads:
http://www.sciencemadness.org/talk/viewthread.php?tid=1089 ("Nitromethane -> Fulminates")
http://www.sciencemadness.org/talk/viewthread.php?tid=16118 ("vicinal amino-nitroalkanes ?")
[Edited on 22-2-2012 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
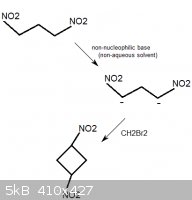
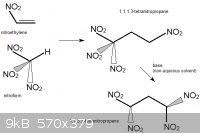
1,1,3,3-tetranitrocyclobutane
1,3-dinitropropane would probably react with methylene bromide, CH2Br2, in the presence of a non-nucleophilic base. This could be a mild base such as
sodium acetate, but another interesting choice could be 1,8-Bis(dimethylamino)naphthalene, sold as "Proton Sponge". The compound has an
extreme affinity for hydrogen ions, yet is not nucleophilic (it is not a source of basic anions that could substitute off the bromine atoms in
CH2Br2).
This would form, besides from some unwanted polymerized byproducts, 1,3-tetranitrocyclobutane.
1,3-tetranitrocyclobutane could be nitrated to 1,1,3,3-tetranitropropane. For example, oxidative nitration of
N-tert-butyl-3-nitroazetidine to N-tert-butyl-3,3-dinitroazetidine can be done in 60% yield with a mixture of sodium nitrite and sodium persulfate in
the presence of potassium ferricyanide, or in 39% yield with a mixture of sodium nitrite and silver nitrate. This oxidative nitration with persulfate
and ferricyanide is probably similar to the type of procedure below:
| Quote: |
To a stirred solution of 2.66 g (66.5 mmoles) of sodium hydroxide in 15 ml of water at 20° was added 5.0 g (66.5 mmoles) of nitroethane. When all the
nitroethane dissolved, the solution was cooled to 5°-7° in an ice-water bath.
The sodium salt of nitroethane was prepared as above. To the stirred solution was added a solution of 20 g (288 mmoles) of sodium nitrite in 50 ml of
water, followed by a solution of 4.4 g (13.4 mmole) of potassium ferricyanide in 25 ml of water. Finally, 16.0 g of solid sodium persulfate (67
mmoles) was added all at once. The reaction temperature, moderated by an ice-water cooling bath, increased to 50°. The orange mixture was stirred at
25° for 1 hour and then cooled to 10°. Urea, 20 g (0.33 mole), was added, followed by 10 ml of glacial acetic acid. The mixture was extracted with
three 25 ml portions of ether and the combined extracts were washed with brine and dried. The crude product was distilled to give 4.2 g (52% yield) of
1,1-dinitroethane, b.p. 87°-89° (16 mm), identified by its nmr spectrum. Patent 4910322
|
Another variation of the reaction may be possible. If 1,1,3,3-tetranitropropane was used instead in the reaction with CH2Br2 and base, then the
1,1,3,3-tetranitropropane could potentially be directly formed, without the need for subsequent nitration.
Information about tetranitropropane
1,1,1,3-tetranitropropane has a melting point around 50 degC. The solid is insoluble in both water and heptane, but soluble in toluene, methanol,
acetic acid, and especially acetone. It has a density of 1.7g/cm3, acceptable thermal stability, and a detonation velocity around 6.75 km/sec.
72g of Nitroethylene is condensed with 150mL trinitromethane, using 300mL methanol as a solvent at 0degC. Next the mixture is refluxed and the excess
methanol is then distilled off with reduced pressure. The residue is dissolved in 0.75L glacial acetic acid, the product (1,1,1,3-tetranitropropane)
then precipitates after addition of water.
In aqueous solutions of alkali or ammonium hydroxide 1,1,3,3-tetranitropropane isomerizes into 1,1,3,3-tetranitropropane (one of the nitro groups
rearranges onto the other carbon). This reaction may seem inexplicable, but in alkanes with geminal or vicinal nitro groups, one of the nitro groups
can potentially ionize off as nitrite. I can write a possible reaction mechanism diagram if anyone really wants to understand how this works. The
reason the 1,1,3,3- isomer forms is probably that it is the most favorable. For example, nitroalkanes that do not have a hydrogen atom on the same
carbon as the dinitro group require a higher temperature for thermal decomposition than those that have such a hydrogen (P. S. DeCarli, D.S.
Ross, Robert Shaw, E. L. Lee, H. D.Stromberg).
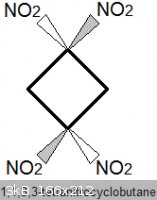
1,1,3,3-tetranitrocyclobutane
Density: 1.83 g/mL. Melting Point: 165 degC (not considered melt-castable, significant decomposition)
This compound is probably more powerful than HMX.
Another explosive compound, TNAZ, for example, which also contains a geminal pair of nitro groups on a square ring, is 7.7% more energetic
than HMX. TNAZ, however, only has three nitro groups so its density is lower, and as a result its detonation velocity is slightly lower.
[Edited on 24-2-2012 by AndersHoveland]
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
| Quote: | 1,1,3,3-tetranitrocyclobutane
Density: 1.83 g/mL. Melting Point: 165 degC (not considered melt-castable, significant decomposition)
This compound is probably more powerful than HMX.
Another explosive compound, TNAZ, for example, which also contains a geminal pair of nitro groups on a square ring, is 7.7% more energetic than HMX.
TNAZ, however, only has three nitro groups so its density is lower, and as a result its detonation velocity is slightly lower. |
this one appeals to me 
BOLD
|
|
|
| Pages:
1
2 |