gl92038
Harmless

Posts: 31
Registered: 14-1-2012
Member Is Offline
Mood: No Mood
|
|
Potassium butanethiolate
Hello, I'm looking for a way to synthesise Potassium butanethiolate.
Actually I have an idea how to do this, but I'm not sure about it.
I found this synthesis
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv4...
and drew this scheme. Can somebody please tell me if it's correct? 
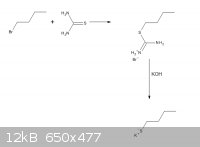
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Potassium bromide is most likely to be formed. I am afraid it can't work.
Rest In Pieces!
|
|
|
gl92038
Harmless

Posts: 31
Registered: 14-1-2012
Member Is Offline
Mood: No Mood
|
|
So, Butane thiol will be the final product?
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
The product before adding the sulphuric acid is the ethane dithiolate in aqueous solution.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
As long as you use the correct stoichiometry, you can get an aqueous solution of potassium butanethiolate, ammonia, and potassium bromide and
(bi)carbonates. To obtain potassium butanethiolate you would have to isolate it somehow. However, if you use a substoichiometric amount of KOH, then
you obtain a solution of S-butylisothiourea which can slowly hydrolyse in water to give n-butylthiol.
Obviously, you might need to adapt the synthesis of the n-butylisothiuronium bromide as it might not efficiently precipitate from ethanol (check the
literature for its most efficient preparation).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
gl92038
Harmless

Posts: 31
Registered: 14-1-2012
Member Is Offline
Mood: No Mood
|
|
Thank you for you replies
|
|
|