Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
benzoin
I debated whether or not it would be of any interest posting the results of my recent benzoin synthesis. But here's a picture of the product. I used
4 year old homemade benzaldehyde and 38% purity KCN as catalyst. The beaker holds 6.3g and the yield was 60.0%. Once you get the precursor and
catalyst the synthesis is quick and easy. This is often the case.
This synthesis is often referred to as the "benzoin condensation." This is a misnomer as it is actually a dimerization.

The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Hohenheim
Harmless

Posts: 9
Registered: 22-2-2012
Location: Stuttgart-Hohenheim
Member Is Offline
Mood: Reborn
|
|
I always appreciate another chemist's experience with a lab, so thanks for this...
...but could you detail this a little better? I mean, what setup did you use, what general conditions, just a classic write-up, you know? I don't mean
to nag, but these are the things that make me want to read and congratulate all the more.
It may not be particularly useful to most people, but I often find that someone will eventually want to know how this is done, and your
experience in detail helps...
Let no man belong to another that can belong to himself.
-Paracelsus Von Hohenheim
Quote from Turd:
"And what is Stugartt supposed to be? Are you sure you don't mean Stuttgart, or is this supposed to be a transcription of some crazy high German
dialect?"
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Interestingly the reaction is also catalysed by thiamin.
A procedure is detailed here;
http://courses.chem.psu.edu/chem35/Syn%20Sp06/35Exp12.pdf
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Interesting that it worked just as well with such an impure KCN.
Quote: Originally posted by Hohenheim  | | ...but could you detail this a little better? I mean, what setup did you use, what general conditions, just a classic write-up, you know?
|
You will hardly find anything more classic than what is already written about it in the year 1921:
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv1...
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by Hohenheim  |
...but could you detail this a little better? I mean, what setup did you use, what general conditions, just a classic write-up, you know?
|
Yes, I will describe the experiment in a little more detail. But, as I said, it is very simple. For the home chemist the real challenge is just
getting the precursors.
I followed the procedure in Brewster (1960). 35mL of ethanol ( I used anhydrous), 10.1 mL benzaldehyde (my old homemade stuff), and 1.5g of KCN (mine
was contaminated with degradation products, I presume KOCN and K2CO3). Since I had titrated it using the Liebig method at 36.7%, I used 4.1g of the
crude reagent.
I mixed all 3 in a 250mL RBF and set up for reflux at a low boil for 40 minutes. This goes quite smoothly except for an occaisional bump. Maybe I
should have added a mag stirrer bar to prevent this. I would mix occaisionally by shaking the whole apparatus. The outlet of the condenser was led
to an inverted funnel in a 600mL beaker containing ~150mL of 10% NaOH. The funnel mouth is placed a slight distance above the liquid level. As
Brewster says there is no HCN generated but just to be cautious a trap is recommended. This way you don't even have to worry about it.
When 40 minutes had elapsed the heat is turned off and the The RBF is stoppered and place in an ice bath. The product has a strong orange color. I
let this set overnight for my convenience.
The next day I scraped out the semi-solid product using a bent spatula onto a 7cm Buchner funnel. The product was then washed successively with 150mL
water, 30mL denatured alcohol, and 20 mL of 25% ether in heptane (I didn't have any pure ether). The product was spread on a piece of paper where it
air dried very quickly.
I treated the filtrate in the Buchner flask as very poisonous, as the cyanide is just a catalyst. It was carefully disposed, wearing full safety
apparel.
This is the Brewster procedure. I took a quick glance at the one in OrgSyn and it seemed very similar.
I will get a melting point today and report it later. I also plan to make benzil from this, and then benzilic acid, per Brewster.
@ScienceSquirrel
Yes, I found out about the use of thiamine from entropy51 also (after my synthesis). That's a very interesting paper. Thanks for posting it.
[Edited on 2-3-2012 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I recrystallized a small portion of my benzoin from boiling denatured alcohol. Nice needles of a very white crystal resulted. The mp was 133-134°C.
My 49th ed CRC (1968-1969) gives the following mp's for benzoin: d =133-134°; l =133-134°; dl =137°. Interesting.
The obvious question: Is one of the stereoisomers favored over the other?
[Edited on 2-3-2012 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  | The mp was 133-134°C. My 49th ed CRC (1968-1969) gives the following mp's for benzoin: d =133-134°; l =133-134°; dl
=137°. Interesting.
The obvious question: Is one of the stereoisomers favored over the other?
|
No. Whenever you have a symmetric left side of the reaction the results on the right side are also symmetric (symmetric in the concept of reactions
means a medium where all possible chiral configurations are represented by same amounts). To achieve asymmetric synthesis (aka enantioselective
synthesis) there has to be some initial asymmetry present (an enantiomeric excess of some reactant, reagent, catalyst or solvent). This is why
symmetric reactions can only give a diastereomeric excess, but no enantiomeric excess.
The reason why the racemate can have a different mp than each of the two enantiomers is in that some racemates form racemic compounds. These have
different physical properties resulting from their different crystal structure (molecular connectivity is the same as for each enantiomer, but the
crystal structure and conformerism is different). The phenomenon is similar to polymorphs where each can have different physical properties resulting
from different crystal structures and molecular conformation even though the molecular configuration is the same in-between all polymorphs of the same
compound (imagine that the mp can differ even for tens of K in some such polymorphs and the dissolution kinetics in the same solvent can differ for
magnitudes!). Thus, there are even cases of dimorphism where a compound can be either a liquid or a crystalline solid at the same conditions.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Today I took the 6g of benzoin I had left and oxidized it to benzil. This was done using con nitric acid (70%). This turns a white alpha-hydroxy
ketone into a yellow diketone:
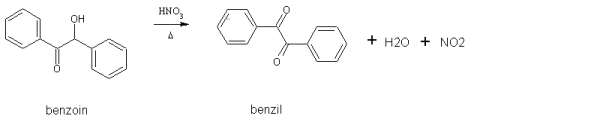
This is another simple preparation. The benzoin and 20mL of acid were added to a 250mL flask and heated for 11 minutes on a steam bath. Per the
recommendation of Brewster I set up an NO2 trap using an inverted funnel over a 10% NaOH solution. This worked quite well. Its effectiveness was
easily monitored due to the strong brown color of the NO2. I could smell just a trace of NO2. During cleanup, however the hood fan was handy for
removing the NO2 trapped in the apparatus.
The tubing shown is Tygon. I know this is not the best material for this application but it was handy and cheap enough to be disposable. Glass would
have been ideal.
The benzil was isolated on a 7cm Buchner funnel, washed with a little water, then a little denatured alcohol, then air dried. The yield was 4.7g, and
the %yield was 75.2%.
 
[Edited on 5-3-2012 by Magpie]
[Edited on 5-3-2012 by Magpie]
[Edited on 6-3-2012 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Bronstein
Harmless

Posts: 41
Registered: 15-1-2007
Member Is Offline
Mood: No Mood
|
|
If anyone is interested, I have tried the benzoin condensation with thiamine as catalyst, following one of the many procedures found on the internet.
It worked very well.
Later I oxidized a portion of it to benzil with copper sulfate/water/NaOH and in situo rearrangement to benzilic acid. (With less NaOH benzil can be
isolated instead). An easy procedure and all chemicals are cheap and easy to get. And good yields.
See http://pubs.acs.org/doi/abs/10.1021/ja01268a017 for the article on it. There is also details on a lot of reductions of benzil in it, you can for
example reduce it to desoxybenzoin with Zn/NH4OH.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
<strong>Magpie</strong>, a full ScienceMadness.org member publication of the process from <a
href="http://en.wikipedia.org/wiki/Benzaldehyde" target="_blank">benzaldehyde</a> <img src="../scipics/_wiki.png" /> through <a
href="http://en.wikipedia.org/wiki/Benzoin" target="_blank">benzoin</a> <img src="../scipics/_wiki.png" /> to <a
href="http://en.wikipedia.org/wiki/Benzil" target="_blank">benzil</a> <img src="../scipics/_wiki.png" /> would be a nice addition to
the existing collection. Organic chemistry hasn't been sufficiently represented.
I do agree that the process is simple, and there's plenty of literature, but we tend to put our own 'amateur' mark on things.
[Edited on 7/9/13 by bfesser]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I agree. We had these same benzaldehyde->benzoin->benzil reactions as lab practice in the undergraduate studies, so that it brings back some
nice memories. That already is reason enough for a contribution to the Prepublication section. Magpie and/or Bronstein, perhaps when you have the time
to do so you could compile some nice report.
The Clemmensen reduction of benzil gives trans-stilbene.
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
<strong>Microscale Organic Experiments</strong> by Kenneth L. Williamson (<a
href="http://www.bookfinder.com/dir/i/Microscale_Organic_Experiments/0669149225/" target="_blank">ISBN 0-669-14922-5</a> <img
src="../scipics/_ext.png" /> has a series of reactions in a section titled
"<Strong>Derivatives of 1,2-Diphenylethane—A Multistep Synthesis</strong>." In this section, the experiments are as follows: has a series of reactions in a section titled
"<Strong>Derivatives of 1,2-Diphenylethane—A Multistep Synthesis</strong>." In this section, the experiments are as follows:
| Quote: | | <em><ol start="54"><li>The Benzoin Condensation: Cyanide Ion and Thiamine
Catalyzed 414</li><li>Nitric Acid Oxidation. Preparation of Benzil from Benzoin. Synthesis of a
Heterocycle: Diphenylquinoxaline 418</li><li>Borohydride Reduction of a Ketone: Hydrobenzoin from
Benzil 422</li><li>1,4-Addition: Reductive Acetylation of
Benzil 426</li><li>Synthesis of an Alkyne from an Alkene. Bromination and Dehydrobromination:
Stilbene and Diphenylacetylene 429</li><li>The Perkin Reaction: Synthesis of
α-Phenylcinnamic Acid 435</li><li>Decarboxylation: Synthesis of
cis-Stilbene 438</li></ol></em> |
By the way, <strong>Magpie</strong>, I love your copper steam bath. If only I had funds when those pop up on eBay...
[editing to include 3<sup>rd</sup> ed. & Pavia <em>et al.</em> to come later]
[Edited on 7/9/13 by bfesser]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by Nicodem  | | We had these same benzaldehyde->benzoin->benzil reactions as lab practice in the undergraduate studies, so that it brings back some nice
memories. That already is reason enough for a contribution to the Prepublication section. |
I agree. My goal is to work all the Brewster procedures that we never had time to do in 1961.  I'll do a writeup for Prepublication after I finish making benzilic acid. I'll do a writeup for Prepublication after I finish making benzilic acid.
Yes, I love it too, and use it whenever possible, even though it is more trouble to set up with the steam generator and all.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Heuteufel
Harmless

Posts: 25
Registered: 5-11-2011
Member Is Offline
Mood: No Mood
|
|
Well done!
I did the thiamin catalysed version some time ago (small scale, because I bought the thiamin in a local drugstore and it wasn´t cheap at all there):
Benzoin Synthesis.
The oxidation was done with copper(II)-sulfate in pyridine:Benzil synthesis
Orgsyn claims, that with nitric acid oxidation is uncomplete, which can be proven by the Fehling's test (Orgsyn synthesis). Do you have still some benzil left to do a Fehling's test?
There are also other ways to perform the oxidation: With copper(II)-acetat and ammonium nitrate, with vanadium(V)-oxychloride and oxygen, ...
Here some interesting synthesis (apart from benzilic acid) that can be done with benzoin-benzil:
Synthesis of the anticonvulsant phenytoin: Phenytoin synthesis
Tetraphenylcyclopentadienone: Tetraphenylcyclopentadienone
A nice demonstration of the formation of a radical anion: Radical anion (If it doesnt work, add a bit (or a lot) more of NaOH. I tried the demonstration, and with the indicated concetration of base, no
colour could be observed. I added more NaOH and then it worked like a charm.)
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Very mpressive. Your investigations put mine to shame, as mine were only cursory.
I did this test and it gave the positive brown with a little red showing the insoluble reduction product Cu2O. Wanting to see what a strong positive
for this test looks like I tested a little fructose. Now that gives a beautiful brick red!
I had just a little benzil left and thought I should get a melting point on it. But when I dissolved it in hot ethanol it seems to have "oiled out."
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
ScienceSquirrel
|
Thread Pruned
13-3-2012 at 05:11 |
niertap
Hazard to Self
 
Posts: 76
Registered: 5-8-2011
Member Is Offline
Mood: hyper-conjucated
|
|
I have researched this reaction quite a bit. (researched with chemicals and elbow grease, not internet and carpel tunnel)
I just did the thiamine catalyzed version. I may publish some time if i get enough time to finish. I made so many flasks of substituted
benzaldehydes.... It's surprising how few of them will give a solid product. I also did some work with the photospectometer on trying to find
the concentration of the yellow deprotinated product of thiamine in different solvents.
I suggest that you oxidize the benzoin to benzil by the base(?) catalyzed reaction of benzoin with benzil in atmospheric oxygen. It turns a really
cool black/purple from the formation of a diradical anion.
Also I believe the optimal synthesis was something like 5ml h20, 15ml HOEt, NaOh to get it to pH 9 (to high and ox, to low and no product) maybe a
half gram of thiamine and 5ml benzaldehyde. The amount of thiamine was way way over kill. The water/EtOH system works really well because it
dissolves benzaldehyde, but benzoin is practically insoluble.
On a side not, one of the derivatives smelled like centipedes and I was very displeased with this. I hate those things. The inch-inch and a half red
ones that snake around really creepily. they also bite.
Ignorance is bliss
Outliers in life are modeled by chemical kinetics
|
|
|