| Pages:
1
2 |
violet sin
International Hazard
    
Posts: 1475
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
so, I searched first. and apologies if this is a repeat for some one.
that being said, I am also interested in phosphoric acid. as I have none in stock and I never really liked purchasing chems over the net. most of the
OTC stuff I find isn't so clean.
1) so is there any way to start w/ TSP(Na3PO4) or bonemeal as my phosphates and end up with a decent clean acid? I had remembered something about
bone meal or phosphate rock can be leached with con. H2SO4 yielding basically gypsum(CaSO4) and a mobile phase of concentrated H3PO4. I brought up the
TSP b/c it looks like nice clean crystal, compared to crushed bones or rock. I could sacrifice some of my purified drain cleaner w/o feeling too bad.
2) also does a home based guy have any prospect for making P2O5 for dessication purposes? I am not trying to make WP with any of these chems so no
worries. I read that it was good for dessication but once hydrated it wasn't easy to regenerate w/o decomp? guess having nice fresh H3PO4 outta the
moisture might not be the worst thing in the world. just I like my dessicators to be regenerative. using CaCl2 mainly.
and realize I am not trying to reinvent the wheel here, just have limited reagents for sure. If it doesn't work I will wait till I can afford to buy
some.
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Mono-Ammonium Phosphate may be converted to Phosphoric Acid (or PPA) via Microwaving under Vacuum.
Repeated dehydration Cycles produce PPA (PolyPhosphoricAcid).
I don't know if P2O5 can be achieved by ordinary methods.
Somewhere in the Science Madness Archives, there are detailed discussions on the matter. Search.
|
|
|
violet sin
International Hazard
    
Posts: 1475
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
Ya found a book to read,.. yay. and came across a quick easy one just like I had mentioned for the acid. though I am pretty sure I won't be making
the P2O5 my self. mainly b/c it revolves around making P4 first, then burning it. no thanks.
I appreciate the info zed, though I am not sure I would know where to get the (NH4)H2PO4 in the first place. the decomp is low temp and vacuum isn't
hard to manage, which is nice. my 12th merck said it's for a leavening agent. though I already have ~10#'s bonemeal for my garden. only $1.25/#
here and I have acid. think I will take that route.
ya I'm new to posting here. been reading for over a month, almost every day. and as it turns out I had not noticed the "next page" button in lower
right on the search engine so I didn't even get to look at all the threads that had pertaining info.. sorry for the quick draw on that last post.
|
|
|
TheVoid
Harmless

Posts: 10
Registered: 5-1-2018
Member Is Offline
Mood: No Mood
|
|
azeotropic distillation
sorry for reviving such an old thread but has anyone tried azeotropic distillation? why wouldnt P-xylene work?
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
See "Phosphoric acid from calcium phosphate" in Prepublication.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Duff
Harmless

Posts: 17
Registered: 10-5-2020
Member Is Offline
|
|
Obligatory apology for necro.
The attached patent claims that distillation of a mixture of phosphoric and acetic acid will result in a phosphoric acid residue with less than 1% by
weight of water. Can anyone comment on the veracity of these claims? It might work since the low boiling point of acetic acid allows it to vaporize
and exit the mixture when heated to 100-110 C, pulling water molecules along with it.
On a side note it is very difficult to find primary source data on the temperatures at which phosphoric acid forms various concentrations of
metaphosphoric acid. I've seen claims ranging from 100-300 C for when phosphoric acid begins to form metaphosphoric acid when heated. I would love to
see some primary source data.
Attachment: US3284171.pdf (346kB)
This file has been downloaded 274 times
[Edited on 12-5-2020 by Duff]
|
|
|
Mateo_swe
National Hazard
   
Posts: 505
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
Earlier in this thread is was said very concentrated phosphoric acid dissolves glass.
I find it strange that this isnt mentioned in the patent where they claim to get 99% phosphoric acid.
Or is this only a problem when one have long time contact between glass and very high concentrations of phosphoric acid?
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Mateo_swe is right, boroglass 3.3 is not resistant against hot conc H3PO4
that's why Magpie suggested vacuum distillation (to avoid hot acid)
sorry this is in my language, you need a translator https://www.verkon.cz/vlastnosti-skla/
here something in eng:
https://www.duran-group.com/en/ueber-duran/duran-eigenschaft...
DURAN® properties
Only hydrofluoric acid, concentrated phosphoric acid and strong alkali cause appreciable surface removal of the glass (glass corrosion) at elevated
temperatures (>100 °C).
|
|
|
Master Triangle
Harmless

Posts: 14
Registered: 24-12-2013
Member Is Offline
Mood: No Mood
|
|
I just found a paper that gives some nice relevant details.
It's got charts of:
>Molecular composition of phosphoric acids over concentration
>H2O vapor pressure over concentration with isotherms
>Bubble point temperature isobars over concentration.
(Also conductivity and viscosity)
Phosphoric Acid and its Interactions with Polybenzimidazole-Type Polymers:
https://link.springer.com/chapter/10.1007/978-3-319-17082-4_...
Also here's a paper that details the freezing of phosphoric acids into hydrated and pure crystalline solids:
https://pubs.acs.org/doi/pdf/10.1021/ie50190a031
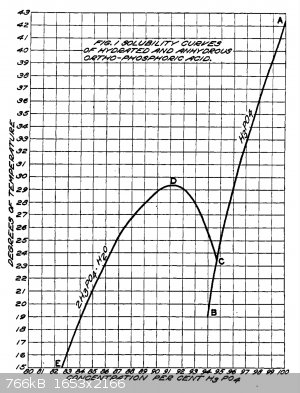
Concentrations up to 95% will not equilibrate to have any appreciable amount of pyrophosphoric acid and the freezing point is depressed to a local
minima of 24C.
This seems to be a good concentration to stop at for most purposes, it's about the limit before you start getting some pyrophosphoric acid, and it is
easy to keep it all liquid (the freezing point increases rapidly past here).
The best way to measure concentration as you boil it down would be with a hydrometer, but you could probably just work out your starting concentration
and then work out water removed by weighing occasionally.
The hydrated solid (2:1 H3PO4:H2O) equates to a 91.5% w/w solution and begins to crystallise out at 29C with a seed crystal.
It seems that many concentrations will easily supercool by a fair bit, putting a little bit in the freezer to get seed crystals should work, and
apparently the crystallisation is very rapid when supercooled and seeded. "The fused anhydrous acid and a solution corresponding to the hydrate were
frequently cooled to almost 0 C. before spontaneous crystallization occurred and both may be kept in a closed vessel indefinitely without
crystallization."
Edit: How do I get an account for the SM wiki? I want to add some of this to the phosphoric acid page.
[Edited on 11-2-2023 by Master Triangle]
|
|
|
chornedsnorkack
National Hazard
   
Posts: 521
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Unfortunately it is paywalled.
Some links that did open:
https://cdnsciencepub.com/doi/pdf/10.1139/v56-102
quotes the endpoint of concentrating, the azeotrope, as 92,1% P2O5 (HPO3 is 88,8%) and boiling at 869 C.
https://isolab.ess.washington.edu/resources/H3PO4.pdf
Vapour composition vs. temperature, in Figure 12 on Page 20 (24 of pdf).
Only up to 800 C, not all the way to azeotrope, and without accompanying liquid concentration.
Figure 14 (page 22, 26 of pdf) gives viscosity up to 118 % phosphoric acid. Unfortunately it gives only the liquid temperatures and does not mark gel
point and glass transition temperatures, nor extend to azeotrope (127%)
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
sci-hub.ru
|
|
|
| Pages:
1
2 |