methylene_beam
Harmless

Posts: 21
Registered: 9-1-2012
Member Is Offline
Mood: No Mood
|
|
diazonium salt formation on heterocycles
I was able to obtain some guanine free base for a good price. I decided to attempt to form the diazonium salt. I followed a standard procedure that
was used on a non heterocycle.
Edit: ref available upon request
| Quote: |
“To a 250 ml RBF there was added 1.0g of guanine(6.6 mmol) 16 mls of water and 4mls of a 50wt% HCl solution. The guanine at first did not go into
solution (the free base is known to be insoluble in water) the addition of the HCl seemed to help (formation of the more soluble salt?) then an ice
bath was added and the temp of the rxn flask was droped to -5c. Sodium nitrite 0.46g (6.7mmol) was added to 4mls of chilled water. This chilled
solution was added over the course of 1 min. after addition the once white solution had taken on a yellow tint (perhaps N02 side product?). This
solution was held for ten mins. KI 1.0g (6.7mmol) was then added in small portions. Right away the solution thickened and changed to a black color
with evolving gas (n2?). Once all the KI was added rxn was stired for 10 mins. Sodium thiosulfate 10wt% was added to the black solution. Addition
immediately cleared up the black solution. The once sludgy appearance had all but dissipated. A yellow precipitate was noted. Solution was filtered to
remove the solids. Solids were washed with thiosulfate but yellow color did not wash out. The yield was 80%. Product solubility seemed to be the same
as guanine. Leading me to believe that the synthesis failed.
|
This is the procedure I followed for the formation of the diazonium salt. I am hesitant to say that this rxn worked because both starting material and
product seem to have the same solubility in IPA.
(before people ask : I don’t have any way of testing melting points)
1) Does my yellow product seem like it could be the desired iodo derative? Remembering that the yellow does not wash out with thiosulfate. (in
previous experiments that failed the yellow would always wash out)
2) I saw gas evolving during the addition of KI this leads me to believe that N2 is leaving.
3) would we expect that both the product and starting material to have similar solubilities?
please post constructive information. not your opinion about what ever and check the egos at the door. If you have experience with a rxn like this I
would love to have some imput on whats going on.
[Edited on 7-3-2012 by methylene_beam]
|
|
|
disulfideprotein
Harmless

Posts: 32
Registered: 14-2-2012
Member Is Offline
Mood: Fractionating
|
|
I dont think you have made it but I could be horribly wrong. Here is my thoughts, un-balanced: diazonium salts decompose above 5C and lose the
nitrogen, and I looked up the process and the nitrous acid part in the equation below is correct.
It does not look like this would be a suitable compound for a diazonium salt. Diazonium salts should look like this R-N2+ X- . If done correctly on
this compound oxygen and nitrogen should have been liberated from the compound. The diazonium salts need to have N2+ at the end. This one has N5O.
What do you think?
C5H5N5O + HCL-->C5H5N5O + NaNO2 + HCl-->C5H5N5O+NaCl+HNO2+KI is this what you did? (Unbalanced...)--> If you did the correct you
should have lost O2 and N2 then went on to this: C6H5N2+ + KI → C6H5I + K+ + N2.
GUANINE FREE BASE:
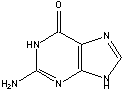
[Edited on 7-3-2012 by disulfideprotein]
You can't arrest me, it was for science!
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Methylene_beam, this is a science forum and you just cited a procedure without giving the reference. Don't you find there is something terribly
unethical in doing so? Please read the forum's posting guidelines and appropriately edit your post above.
|
|
|
methylene_beam
Harmless

Posts: 21
Registered: 9-1-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | | Methylene_beam, this is a science forum and you just cited a procedure without giving the reference. Don't you find there is something terribly
unethical in doing so? Please read the forum's posting guidelines and appropriately edit your post above. |
http://www.webassign.net/sample/ncsumeorgchem2/lab_9/manual....
now that I gave you the ref nicodem will my substrate work.
[Edited on 9-3-2012 by methylene_beam]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The experiment you quoted did not came from the above site. A different Sandmeyer reaction is described there (synthesis of ethyl p-iodobenzoate).
Does that mean that the quote is not a real quote, but is the description of your experiment? If so, why are you playing this hide and seek game about
references? It is only exhausting and does not help you in any way to obtain the information you seek.
A short literature search shows that the conclusions from that quote - in regard to the failed experiment - are most likely correct or partially
correct (without any analytical data it impossible to give you an answer).
I could find only two examples of the Sandmeyer reaction of guanine to give 2-iodo-6-hydroxylpurine (I assume you want to prepare this compound, is
that right?). One article (Yingyong Huaxue, 23, 1156-1160) is written in Chinese and I only have access to its Chemical Abstract
147, 427138 where a nitrosation of guanine with NaNO2 in H2SO4(aq) at 0 °C (1.5 h) followed by KI at rt (1 h) is mentioned to give a 53% yield
of 2-iodo-6-hydroxylpurine (without further details). The other is luckily in English and freely accessible (DOI 10.5012/jkcs.2010.54.4.429). It
describes a Sandmeyer reaction of guanine using over-stoichiometric amounts of CuI to give 2-iodo-6-hydroxylpurine in 57% yield. There are also other
related transformations of guanine described there that might be of interest to you, so I'm sure you will find it worth reading.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|