Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Preparation of Benzoin
by Magpie
March 7, 2012
A. Introduction
This procedure is for the preparation of 6-8g of benzoin. It was taken from Brewster (ref 1). The procedure is quick, easy, and gives a decent
yield. The only tricky part is the handling of the catalyst KCN, a deadly poison. Be advised that there is a procedure for benzoin that utilizes a
thiamine catalyst instead (ref 2).
This synthesis is often referred to as the “benzoin condensation.” However, this is a misnomer as it is actually a dimerization. That is, 2
molecules of benzaldehyde react to form one molecule of benzoin, with no expulsion of a small molecule:
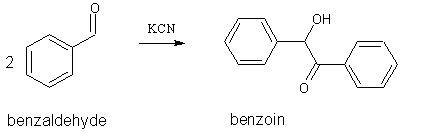
The cyanide ion is a catalyst for this reaction, having just the right balance of nucleophilicity to attack the carbonyl carbon, stabilize the
intermediate anion, then provide a satisfactory leaving group at the end of the reaction (ref 2). For the complete mechanism see reference 2.
B. Procedure
1. Chemicals
10.1 mL (0.1 mole) benzaldehyde
1.5g KCN (see note 1)
35 mL ethanol
16.7g NaOH
30 mL denatured alcohol
10 mL of ether (see note 2)

CAUTION! KCN is a deadly poison and must be handled with the utmost care. In no case should it be in an acid medium as this will
result in the release of the poisonous gas HCN.
2. Equipment
250mL RBF
condenser & cooling water supply
7cm lab funnel
600mL beaker
~ 1 ft of ¼” rubber tubing
plug w/tublature
heating mantle
7cm Büchner funnel
ringstand
2 clamps
1-hole rubber stopper
ice-bath
stopper for RBF
3. Setting Up the Apparatus
Set up the heating mantle, 250mL RBF, and the condenser for reflux. Although, as Brewster says, there is no HCN generated by the above reaction, use
of a safety absorbtion trap is recommended. This is accomplished by using the rubber hose to lead from the top of the condenser to an inverted
funnel. Attach the hose to a plug w/tublature installed at the top of the condenser. The trap is made up using about 150mL of 10wt% NaOH placed in a
600 mL beaker. The mouth of the inverted funnel is located ~1cm above the liquid surface. The funnel is held in place using a 1-hole stopper and
clamp. See the picture below.

benzoin apparatus
4. Conducting the Reaction
Add the ethanol, benzaldehyde, and KCN to the 250mL RBF. Attach the reflux condenser and clamp the glassware to the ringstand with the heating mantle
in place. Turn on the condenser water pump (cold water) and supply sufficient heat to the RBF to keep the reactants at a low boil for 40 minutes. As
the reaction proceeds the color of my reactants turned to a yellow then to a deep orange. After 40 minutes has elapsed, turn off the heat, let the
RBF cool somewhat, stopper, and then place in an ice-bath. Always keep in mind that this flask contains a deadly poison, as the cyanide is only a
catalyst and remains unchanged.
5. Workup
Set up a 7cm Büchner funnel with a filter paper for vacuum filtration. When the benzoin appears to be fully crystallized wash it onto the Buchner
funnel using 150mL of water. Follow this by washing the benzoin with 30mL of denatured alcohol, then 10 mL of ether (note 2). Place the crystals on
a piece of paper to dry.
The filtrate should be treated with the same caution as before as it now contains the full aliquot of KCN.
6. Waste Disposal
Cyanide in the waste water can be converted to the relatively innocuous cyanate using sodium hypochlorite, ie,
CN- + OCl- ----> OCN- + Cl-
A 50% molar excess of Clorox (5.25% NaOCl) is added and the mixture is allowed to stand at room temperature for several hours (ref 3). The Prussian
Blue test can be used to verify the destruction of the cyanide ion.
C. Yield
Per Brewster the expected yield is 7-8g. My yield was 6.3g for a % yield of 60.0%.

6.3g of benzoin
D. Melting Point Determination
Recrystallize a small portion of the benzoin from hot ethanol (denatured is ok) for observation of its melting point (137°C, ref 4).

melting point apparatus
E. Discussion
The benzoin, an α-hydroxy ketone, can be easily oxidized to benzil, an α-diketone, using nitric acid. This in turn can be converted to the
ester potassium benzilate through a rearrangement using KOH. This can then be converted to benzilic acid using HCl. Procedures for these syntheses
can also be found in reference 1.
Notes
1. My KCN purity was 36.7%. Therefore I used 4.1g to provide the full 1.5g of KCN required.
2. Not having any pure ether I substituted 30mL of 25% ether in heptane.
F. References
1. Unitized Experiments in Organic Chemistry, 1960, by Brewster et al.
2. http://courses.chem.psu.edu/chem35/Syn%20Sp06/35Exp12.pdf (Thanks to ScienceSquirrel for this reference.)
3. Prudent Practices for Disposal of Chemicals from Laboratories, 1983, p. 87, National Research Council et al.
4. Handbook of Chemistry and Physics, CRC (1968-1969), 49th ed.
[Edited on 8-3-2012 by Magpie]
[Edited on 8-3-2012 by Magpie]
[Edited on 8-3-2012 by Magpie]
[Edited on 8-3-2012 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Very nice writeup, Magpie. I think it would be nice to add a section detailing the recovery or destruction of the cyanide reagent and the absorbtion
solution. Which did you do when you actually carried out this synthesis?
|
|
|
zoombafu
Hazard to Others
  
Posts: 255
Registered: 21-11-2011
Location: U.S.
Member Is Offline
Mood: sciencey
|
|
This is a very nice lab write up. Good job. You should probably add a section in the disposal of the left over reagents.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Thank you. A section was added on waste cyanide disposal.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I knew there was something familiar about the dimerization of benzaldehyde to form benzoin: it reminds me of the Canizzaro reaction. In both cases
one carbonyl group is oxidized while the other is reduced. So, could this reaction be called a "disproportionated dimerization"?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
I like the write up Magpie, I have run this reaction multiple times on approximately 100 grams of benzaldehyde at a time, weighing out the potassium
cyanide was scary due to the amount of the catalyst required on this scale. The reaction goes like a champ with freshly distilled benzaldehyde.
Crystallization of a flask full of benzoin on this scale by adding a seed crystal is violently exothermic as described in Organic Syntheses. I am
converting the benzoin to trans-stilbene via a clemmesen reduction, I then brominated it to produce meso-stilbene dibromide which will subsequently
converted into diphenylacetylene for future chemistry projects such as the synthesis of tetraphenylcyclobutadiene complexes of transition metals and
hexaphenylbenzene. Most of this chemistry is discussed in the early collective volumes of Organic Syntheses or in Fieser's organic chemistry
experiment books.
The mechanism of the benzoin condensation involves the formation of benzaldehyde cyanohydrin, the alpha carbon of this is then deprotonated to form a
stabilized carbanion, which then attacks another molecule of benzaldehyde. The alkoxide anion formed is then protonated and the tetrahedral
cyanohydrin intermediate collapses to form benzoin and the cyanide anion again.
The thiamine benzoin condensation can be run with thiamine extracted from thiamine hydrochloride tablets. Fresh tablets must be used from my
experience for dependable results.
[Edited on 11-3-2012 by benzylchloride1]
Amateur NMR spectroscopist
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
<strong>benzylchloride1</strong>, you switched tenses a few times in your post. You have done your reaction scheme, or you plan to? What
step are you at?
<strong>Magpie</strong>, I love the photograph of your <a href="http://en.wikipedia.org/wiki/Thiele_tube" target="_blank">Thiele
tube</a> <img src="../scipics/_wiki.png" /> melting point apparatus. Very clever to include the silicone oil bottle in the photo. From
what source did you get the propane regulator? I've seen similar ones for sale from Avogadro's on eBay, but they're pricey. I currently use one that
I built from surplus shop parts.
[Edited on 7/9/13 by bfesser]
|
|
|
ItalianChemist
Hazard to Others
  
Posts: 172
Registered: 26-1-2011
Location: Italy
Member Is Online
Mood: No Mood
|
|
Very nice work!
I've tried thiamine method and I obtained a 57% yield!
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by bfesser  |
From what source did you get the propane regulator? I've seen similar ones for sale from Avogadro's on eBay, but they're pricey. I currently use one
that I built from surplus shop parts.
[Edited on 3/11/12 by bfesser] |
I picked that up at a Ranch & Home store in their large BBQ equipment section. I think it was around $15.
Yes, I like to use silicone oil as it is non-flammable, although expensive. The brand shown in the picture had a purple dye but I removed that with
activated charcoal.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
| Quote: |
benzylchloride1, you switched tenses a few times in your post. You have done your reaction scheme, or you plan to? What step are you at?
|
I have material at multiple stages, most of the material is currently at the benzoin stage, I have made a few grams of benzil and
tetraphenylcyclopentadienone. I ran the Clemmensen reduction on 25 grams of benzoin and this reaction gave a 45% yield after several
recrystallizations at this scale. Bromination with pyridinium perbromide as described by Fieser gave a high yield of the stilbene dibromide. I pushed
most of the trans-stilbene to the dibromide stage, a small sample was saved for IR and NMR. I have not run the double dehydrohalogenation to form the
diphenylacetylene yet. I plan on making around a gram of hexaphenylbenzene; the rest of the diphenylacetylene will be used for organometallic
chemistry.
[Edited on 11-3-2012 by benzylchloride1]
Amateur NMR spectroscopist
|
|
|