gl92038
Harmless

Posts: 31
Registered: 14-1-2012
Member Is Offline
Mood: No Mood
|
|
Phenyl p-dioxane synthesis
After I saw this synthesis ( http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv4... ) I came up with the idea of making phenyl p-dioxane but I'm not sure if my route
is correct. So could you please correct me if I'm wrong.
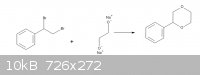
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
sometimes bromoalkanes can react with a strong base to pull out an HBr rather than substitute a hydroxyl group.
For example, bromocyclohexane can react with KOH to form cyclohexene.
http://d.web.umkc.edu/drewa/Chem321L/Handouts/Lab9E2Cyclohex...
from wiki...
| Quote: |
Dehydrohalogenation of Haloalkanes
Dehydrohalogenation is a very common method for creating alkenes. It uses the E2 elimination mechanism. The base used is generally a strong base such
as KOH (potassium hydroxide) or NaOCH3 (sodium methoxide). The haloalkane must have a hydrogen and halide 180° from each other on neighboring
carbons. If there is no hydrogen 180° from the halogen on a neighboring carbon, the reaction will not take place.
Dehalogenation of Vicinal Dibromides
The dehalogenation of vicinal dihalides (halides on two neighboring carbons) is another method for synthesizing alkenes. The reaction can take place
using either sodium iodide in a solution of acetone, or it can be performed using zinc dust in a solution of either heated ethanol or acetic acid.
|
[Edited on 18-3-2012 by AndersHoveland]
|
|
|
kavu
Hazard to Others
  
Posts: 207
Registered: 11-9-2011
Location: Scandinavia
Member Is Offline
Mood: To understand is to synthesize
|
|
Sulphur is a much softer nucleophile and less basic than oxygen, the route you presented would probably lead to a gunky mixture of all sorts of
things. In an article by R. K. Summerbell and L. N. Bauer J. Am. Chem. Soc., 1935, 57 (12), pp 2364–2368 a viable home chemistry method for the
preparation of phenyl p-dioxane is presented. PhMgBr is coupled with monochloro-p-dioxane, prepared by addition of HCl to p-dioxene.
[Edited on 18-3-2012 by kavu]
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Interestingly the picture shown in the Org Syn prep you link to does not match their procedure (picture shows sodium phenoxide while the described
prep uses elemental sodium). First time I've seen an error like that in Org Syn (or maybe I'm confused).
As for your route, I think it could work, but a route is not a procedure. You might need a lot of trial and error to achieve any kind of success. I
notice that the Org Syn prep you reference uses 2 liters of ethanol for about 20ml of each of the other two reagents (100:1:1 by volume, more or
less), which looks like an attempt to maximize the proportion of intramolecular reactions via dilution.
The less you bet, the more you lose when you win.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
In the present case you describe, Anders is right...there is a big chance that instead of a cyclisation product you get an elimination or even a
bis-elimination...straight to phenylacetylen...via an intermediary alfa- or beta- bromostyren...
C6H5-CHBr-CH2Br + NaO-CH2-CH2-ONa --> C6H5-CBr=CH2 + NaBr + HO-CH2-CH2-ONa
C6H5-CBr=CH2 + HO-CH2-CH2-ONa --> C6H5-C#C-H + NaBr + HOCH2-CH2OH
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Indeed, there appears not to exist a slightest chance that 1-phenyl-1,2-dibromoethane could react with something so basic as the bis-deprotonated
ethylene glycol. A cursory search of the forum would give evidence of this.
2-Phenyl-1,4-dioxane is most easily prepared via the usual route for 1,4-dioxanes, that is by acid catalysed cyclisation of the appropriate diol, the
2-(1-hydroxy-1-phenylethoxy)ethanol. This compound should be easily accessible by the base catalysed solvolysis of styrene oxide in ethylene glycol.
A more straightforward approach is the direct sulfuric acid catalysed reaction of styrene oxide with ethylene glycol, which under appropriate
conditions gives 2-phenyl-1,4-dioxane as the major isolable product (see DOI: 10.1021/jo01093a039 for the procedure and further details). Admittedly,
the yield is only moderate and might be lower in comparison to the equivalent two step process, but it avoids the isolation of the water soluble
2-(1-hydroxy-1-phenylethoxy)ethanol. Styrene oxide is easily made from styrene.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
gl92038
Harmless

Posts: 31
Registered: 14-1-2012
Member Is Offline
Mood: No Mood
|
|
Thank you all for your replies, especially for the last 2 
I'll try the described method with styrene oxide
|
|
|